Sensory Neurons Co-opt Classical Immune Signaling Pathways to Mediate Chronic Itch
- PMID: 28890086
- PMCID: PMC5658016
- DOI: 10.1016/j.cell.2017.08.006
Sensory Neurons Co-opt Classical Immune Signaling Pathways to Mediate Chronic Itch
Abstract
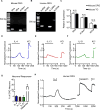
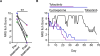
Mammals have evolved neurophysiologic reflexes, such as coughing and scratching, to expel invading pathogens and noxious environmental stimuli. It is well established that these responses are also associated with chronic inflammatory diseases, including asthma and atopic dermatitis. However, the mechanisms by which inflammatory pathways promote sensations such as itch remain poorly understood. Here, we show that type 2 cytokines directly activate sensory neurons in both mice and humans. Further, we demonstrate that chronic itch is dependent on neuronal IL-4Rα and JAK1 signaling. We also observe that patients with recalcitrant chronic itch that failed other immunosuppressive therapies markedly improve when treated with JAK inhibitors. Thus, signaling mechanisms previously ascribed to the immune system may represent novel therapeutic targets within the nervous system. Collectively, this study reveals an evolutionarily conserved paradigm in which the sensory nervous system employs classical immune signaling pathways to influence mammalian behavior.
Keywords: IL-13; IL-4; IL-4Rα; JAK1; atopic dermatitis; itch; pruriceptor; pruritus; type 2 cytokines.
Copyright © 2017 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures

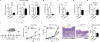
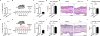

Comment in
-
Neuroimmunology: JAK in the itch.Nat Rev Immunol. 2017 Oct;17(10):591. doi: 10.1038/nri.2017.114. Epub 2017 Sep 18. Nat Rev Immunol. 2017. PMID: 28920589 No abstract available.
-
Neuroimmunology: Scratching the surface of chronic itch.Nat Rev Neurosci. 2017 Nov;18(11):642. doi: 10.1038/nrn.2017.126. Epub 2017 Oct 5. Nat Rev Neurosci. 2017. PMID: 28978978 No abstract available.
-
Neuroimmunological disorders: JAK in the itch.Nat Rev Drug Discov. 2017 Oct 30;16(11):753. doi: 10.1038/nrd.2017.204. Nat Rev Drug Discov. 2017. PMID: 29081518 No abstract available.
References
-
- Agarwal N, Offermanns S, Kuner R. Conditional gene deletion in primary nociceptive neurons of trigeminal ganglia and dorsal root ganglia. Genesis. 2004;38:122–129. - PubMed
-
- Artis D, Spits H. The biology of innate lymphoid cells. Nature. 2015;517:293–301. - PubMed
-
- Beck LA, Thaçi D, Hamilton JD, Graham NM, Bieber T, Rocklin R, Ming JE, Ren H, Kao R, Simpson E, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med. 2014;371:130–139. - PubMed
MeSH terms
Substances
Grants and funding
- K08 AR065577/AR/NIAMS NIH HHS/United States
- R01 GM101218/GM/NIGMS NIH HHS/United States
- R01 AR070116/AR/NIAMS NIH HHS/United States
- R56 AR064294/AR/NIAMS NIH HHS/United States
- T32 HL007317/HL/NHLBI NIH HHS/United States
- R01 DA037261/DA/NIDA NIH HHS/United States
- R01 AI077600/AI/NIAID NIH HHS/United States
- R01 NS042595/NS/NINDS NIH HHS/United States
- R01 AR056318/AR/NIAMS NIH HHS/United States
- P30 CA091842/CA/NCI NIH HHS/United States
- R01 DK103901/DK/NIDDK NIH HHS/United States
- UL1 TR000448/TR/NCATS NIH HHS/United States
- R01 AI125743/AI/NIAID NIH HHS/United States
- R21 AR068012/AR/NIAMS NIH HHS/United States
- P30 DK052574/DK/NIDDK NIH HHS/United States
- UL1 TR002345/TR/NCATS NIH HHS/United States
- R01 EY024704/EY/NEI NIH HHS/United States
- S10 RR027552/RR/NCRR NIH HHS/United States
- R01 NS094344/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

