A key role for IL-7R in the generation of microenvironments required for thymic dendritic cells
- PMID: 28890536
- PMCID: PMC5698111
- DOI: 10.1038/icb.2017.74
A key role for IL-7R in the generation of microenvironments required for thymic dendritic cells
Abstract
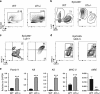
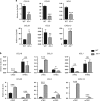
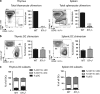
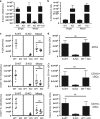
Interleukin-7 receptor (IL-7R) signaling is critical for multiple stages of T-cell development, but a role in the establishment of the mature thymic architecture needed for T-cell development and thymocyte selection has not been established. Crosstalk signals between developing thymocytes and thymic epithelial cell (TEC) precursors are critical for their differentiation into cortical TECs (cTECs) and medullary TECs (mTECs). In addition, mTEC-derived factors have been implicated in the recruitment of thymic dendritic cells (DCs) and intrathymic DC development. We therefore examined corticomedullary structure and DC populations in the thymus of Il7r-/- mice. Analysis of TEC phenotype and spatial organization revealed a striking shift in the mTEC to cTEC ratio, accompanied by disorganized corticomedullary structure. Several of the thymic subsets known to have DC potential were nearly absent, accompanied by reductions in DC cell numbers. We also examined chemokine expression in the Il7r-/- thymus, and found a significant decrease in mTEC-derived CCR7 ligand expression, and high levels of cTEC-derived chemokines, including CCL25 and CXCL12. Although splenic DCs were similarly affected, bone marrow (BM) precursors capable of giving rise to DCs were unperturbed. Finally, BM chimeras showed that there was no intrinsic need for IL-7R signaling in the development or recruitment of thymic DCs, but that the provision of wild-type progenitors enhanced reconstitution of thymic DCs from Il7r-/- progenitors. Our results are therefore supportive of a model in which Il7r-dependent cells are required to set up the microenvironments that allow accumulation of thymic DCs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Anderson G, Takahama Y. Thymic epithelial cells: working class heroes for T cell development and repertoire selection. Trends Immunol 2012; 33: 256–263. - PubMed
-
- Ouabed A, Hubert FX, Chabannes D, Gautreau L, Heslan M, Josien R. Differential control of T regulatory cell proliferation and suppressive activity by mature plasmacytoid versus conventional spleen dendritic cells. J Immunol 2008; 180: 5862–5870. - PubMed
-
- Donskoy E, Goldschneider I. Two developmentally distinct populations of dendritic cells inhabit the adult mouse thymus: demonstration by differential importation of hematogenous precursors under steady state conditions. J Immunol 2003; 170: 3514–3521. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

