Outer Membrane Vesicles Prime and Activate Macrophage Inflammasomes and Cytokine Secretion In Vitro and In Vivo
- PMID: 28890719
- PMCID: PMC5574916
- DOI: 10.3389/fimmu.2017.01017
Outer Membrane Vesicles Prime and Activate Macrophage Inflammasomes and Cytokine Secretion In Vitro and In Vivo
Abstract
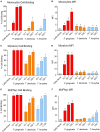
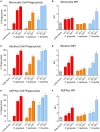
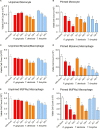
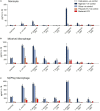
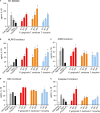
Outer membrane vesicles (OMVs) are proteoliposomes blebbed from the surface of Gram-negative bacteria. Chronic periodontitis is associated with an increase in subgingival plaque of Gram-negative bacteria, Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia. In this study, we investigated the immune-modulatory effects of P. gingivalis, T. denticola, and T. forsythia OMVs on monocytes and differentiated macrophages. All of the bacterial OMVs were phagocytosed by monocytes, M(naïve) and M(IFNγ) macrophages in a dose-dependent manner. They also induced NF-κB activation and increased TNFα, IL-8, and IL-1β cytokine secretion. P. gingivalis OMVs were also found to induce anti-inflammatory IL-10 secretion. Although unprimed monocytes and macrophages were resistant to OMV-induced cell death, lipopolysaccharide or OMV priming resulted in a significantly reduced cell viability. P. gingivalis, T. denticola, and T. forsythia OMVs all activated inflammasome complexes, as monitored by IL-1β secretion and ASC speck formation. ASC was critical for OMV-induced inflammasome formation, while AIM2-/- and Caspase-1-/- cells had significantly reduced inflammasome formation and NLRP3-/- cells exhibited a slight reduction. OMVs were also found to provide both priming and activation of the inflammasome complex. High-resolution microscopy and flow cytometry showed that P. gingivalis OMVs primed and activated macrophage inflammasomes in vivo with 80% of macrophages exhibiting inflammasome complex formation. In conclusion, periodontal pathogen OMVs were found to have significant immunomodulatory effects upon monocytes and macrophages and should therefore influence pro-inflammatory host responses associated with disease.
Keywords: Porphyromonas gingivalis; Tannerella forsythia; Treponema denticola; inflammasomes; macrophages; outer membrane vesicles; periodontitis.
Figures





References
-
- O’Brien-Simpson N, Pathirana R, Walker G, Reynolds E. Porphyromonas gingivalis RgpA-Kgp proteinase-adhesin complexes penetrate gingival tissue and induce proinflammatory cytokines or apoptosis in a concentration-dependent manner. Infect Immun (2009) 77:1246–61. 10.1128/IAI.01038-08 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

