The Biphasic Root Growth Response to Abscisic Acid in Arabidopsis Involves Interaction with Ethylene and Auxin Signalling Pathways
- PMID: 28890725
- PMCID: PMC5574904
- DOI: 10.3389/fpls.2017.01493
The Biphasic Root Growth Response to Abscisic Acid in Arabidopsis Involves Interaction with Ethylene and Auxin Signalling Pathways
Abstract
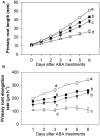
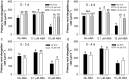
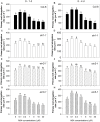
Exogenous abscisic acid (ABA) is known to either stimulate or inhibit root growth, depending on its concentration. In this study, the roles of ethylene and auxin in this biphasic effect of ABA on root elongation were investigated using chemical inhibitors and mutants. Inhibitors of ethylene perception and biosynthesis and an auxin influx inhibitor were all found to block the inhibitory effect of high ABA concentrations, but not the stimulatory effect of low ABA concentrations. In addition, three ethylene-insensitive mutants (etr1-1, ein2-1, and ein3-1), two auxin influx mutants (aux1-7, aux1-T) and an auxin-insensitive mutant (iaa7/axr2-1) were all insensitive to the inhibitory effect of high ABA concentrations. In the case of the stimulatory effect of low ABA concentrations, it was blocked by two different auxin efflux inhibitors and was less pronounced in an auxin efflux mutant (pin2/eir1-1) and in the iaa7/axr2-1 auxin-insensitive mutant. Thus it appears that the stimulatory effect seen at low ABA concentrations is via an ethylene-independent pathway requiring auxin signalling and auxin efflux through PIN2/EIR1, while the inhibitory effect at high ABA concentrations is via an ethylene-dependent pathway requiring auxin signalling and auxin influx through AUX1.
Keywords: Arabidopsis; abscisic acid (ABA); auxin signalling; auxin transport; ethylene biosynthesis; ethylene signalling; hormone; root elongation.
Figures


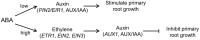
References
-
- Alonso J. M., Stepanova A. N., Solano R., Wisman E., Ferrari S., Ausubel F. M., et al. (2003b). Five components of the ethylene-response pathway identified in a screen for weak ethylene-insensitive mutants in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 100 2992–2997. 10.1073/pnas.0438070100 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

