NF-κB regulates GDF-15 to suppress macrophage surveillance during early tumor development
- PMID: 28891811
- PMCID: PMC5617672
- DOI: 10.1172/JCI91561
NF-κB regulates GDF-15 to suppress macrophage surveillance during early tumor development
Abstract
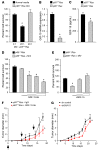
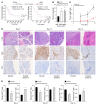
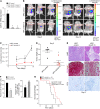
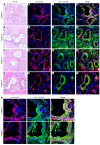
Macrophages are attracted to developing tumors and can participate in immune surveillance to eliminate neoplastic cells. In response, neoplastic cells utilize NF-κB to suppress this killing activity, but the mechanisms underlying their self-protection remain unclear. Here, we report that this dynamic interaction between tumor cells and macrophages is integrally linked by a soluble factor identified as growth and differentiation factor 15 (GDF-15). In vitro, tumor-derived GDF-15 signals in macrophages to suppress their proapoptotic activity by inhibiting TNF and nitric oxide (NO) production. In vivo, depletion of GDF-15 in Ras-driven tumor xenografts and in an orthotopic model of pancreatic cancer delayed tumor development. This delay correlated with increased infiltrating antitumor macrophages. Further, production of GDF-15 is directly regulated by NF-κB, and the colocalization of activated NF-κB and GDF-15 in epithelial ducts of human pancreatic adenocarcinoma supports the importance of this observation. Mechanistically, we found that GDF-15 suppresses macrophage activity by inhibiting TGF-β-activated kinase (TAK1) signaling to NF-κB, thereby blocking synthesis of TNF and NO. Based on these results, we propose that the NF-κB/GDF-15 regulatory axis is important for tumor cells in evading macrophage immune surveillance during the early stages of tumorigenesis.
Conflict of interest statement
Figures


Similar articles
-
Inhibition of constitutive NF-kappa B activity by I kappa B alpha M suppresses tumorigenesis.Oncogene. 2003 Mar 6;22(9):1365-70. doi: 10.1038/sj.onc.1206323. Oncogene. 2003. PMID: 12618762
-
Metformin-treated cancer cells modulate macrophage polarization through AMPK-NF-κB signaling.Oncotarget. 2017 Mar 28;8(13):20706-20718. doi: 10.18632/oncotarget.14982. Oncotarget. 2017. PMID: 28157701 Free PMC article.
-
Euscaphic acid isolated from roots of Rosa rugosa inhibits LPS-induced inflammatory responses via TLR4-mediated NF-κB inactivation in RAW 264.7 macrophages.J Cell Biochem. 2012 Jun;113(6):1936-46. doi: 10.1002/jcb.24062. J Cell Biochem. 2012. PMID: 22234926
-
Immunosurveillance of pancreatic adenocarcinoma: insights from genetically engineered mouse models of cancer.Cancer Lett. 2009 Jun 28;279(1):1-7. doi: 10.1016/j.canlet.2008.09.037. Epub 2008 Nov 14. Cancer Lett. 2009. PMID: 19013709 Review.
-
Non-canonical NF-κB signaling activation and regulation: principles and perspectives.Immunol Rev. 2011 Nov;244(1):44-54. doi: 10.1111/j.1600-065X.2011.01059.x. Immunol Rev. 2011. PMID: 22017430 Review.
Cited by
-
LPS induces rapid increase in GDF15 levels in mice, rats, and humans but is not required for anorexia in mice.Am J Physiol Gastrointest Liver Physiol. 2022 Feb 1;322(2):G247-G255. doi: 10.1152/ajpgi.00146.2021. Epub 2021 Dec 22. Am J Physiol Gastrointest Liver Physiol. 2022. PMID: 34935522 Free PMC article.
-
Growth/Differentiation Factor-15 (GDF-15): From Biomarker to Novel Targetable Immune Checkpoint.Front Immunol. 2020 May 19;11:951. doi: 10.3389/fimmu.2020.00951. eCollection 2020. Front Immunol. 2020. PMID: 32508832 Free PMC article. Review.
-
Macrophage Responses to Environmental Stimuli During Homeostasis and Disease.Endocr Rev. 2021 Jul 16;42(4):407-435. doi: 10.1210/endrev/bnab004. Endocr Rev. 2021. PMID: 33523133 Free PMC article.
-
Microenvironmental modulation of the developing tumour: an immune-stromal dialogue.Mol Oncol. 2021 Oct;15(10):2600-2633. doi: 10.1002/1878-0261.12773. Epub 2020 Aug 28. Mol Oncol. 2021. PMID: 32741067 Free PMC article. Review.
-
Preoperative inflammatory markers of NLR and PLR as indicators of poor prognosis in resectable HCC.PeerJ. 2019 Oct 14;7:e7132. doi: 10.7717/peerj.7132. eCollection 2019. PeerJ. 2019. PMID: 31632844 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

