Sitagliptin and Roux-en-Y gastric bypass modulate insulin secretion via regulation of intra-islet PYY
- PMID: 28892258
- PMCID: PMC5836881
- DOI: 10.1111/dom.13113
Sitagliptin and Roux-en-Y gastric bypass modulate insulin secretion via regulation of intra-islet PYY
Abstract
Aims: The gut hormone peptide tyrosine tyrosine (PYY) is critical for maintaining islet integrity and restoring islet function following Roux-en-Y gastric bypass (RYGB). The expression of PYY and its receptors (NPYRs) in islets has been documented but not fully characterized. Modulation of islet PYY by the proteolytic enzyme dipeptidyl peptidase IV (DPP-IV) has not been investigated and the impact of DPP-IV inhibition on islet PYY function remains unexplored. Here we have addressed these gaps and their effects on glucose-stimulated insulin secretion (GSIS). We have also investigated changes in pancreatic PYY in diabetes and following RYGB.
Methods: Immunohistochemistry and gene expression analysis were used to assess PYY, NPYRs and DPP-IV expression in rodent and human islets. DPP-IV activity inhibition was achieved by sitagliptin. Secretion studies were used to test PYY and the effects of sitagliptin on insulin release, and the involvement of GLP-1. Radioimmunoassays were used to measure hormone content in islets.
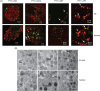
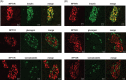
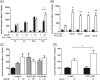
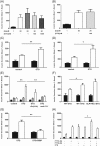
Results: PYY and DPP-IV localized in different cell types in islets while NPYR expression was confined to the beta-cells. Chronic PYY application enhanced GSIS in rodent and diabetic human islets. DPP-IV inhibition by sitagliptin potentiated GSIS; this was mediated by locally-produced PYY, and not GLP-1. Pancreatic PYY was markedly reduced in diabetes. RYGB strongly increased islet PYY content, but did not lead to full restoration of pancreatic GLP-1 levels.
Conclusion: Local regulation of pancreatic PYY, rather than GLP-1, by DPP-IV inhibition or RYGB can directly modulate the insulin secretory response to glucose, indicating a novel role of pancreatic PYY in diabetes and weight-loss surgery.
Keywords: Roux-En-Y gastric bypass; dipeptidyl peptidase IV; glucagon-like peptide-1; insulin; islets; peptide tyrosine tyrosine.
© 2017 The Authors. Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Conflict of interest statement
No potential conflicts of interest relevant to this article were reported.
Figures


References
-
- Sam AH, Gunner DJ, King A, et al. Selective ablation of peptide YY cells in adult mice reveals their role in beta cell survival. Gastroenterology. 2012;143:459–468. - PubMed
-
- Khan D, Vasu S, Moffett RC, Irwin N, Flatt PR. Islet distribution of Peptide YY and its regulatory role in primary mouse islets and immortalised rodent and human beta‐cell function and survival. Mol Cell Endocrinol. 2016;436:102–113. - PubMed
-
- Shi YC, Loh K, Bensellam M, et al. Pancreatic PYY is critical in the control of insulin secretion and glucose homeostasis in female mice. Endocrinology. 2015;156:3122–3136. - PubMed
-
- Persaud SJ, Bewick GA. Peptide YY: more than just an appetite regulator. Diabetologia. 2014;57:1762–1769. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

