Phase Segregation in Cs-, Rb- and K-Doped Mixed-Cation (MA)x(FA)1-xPbI3 Hybrid Perovskites from Solid-State NMR
- PMID: 28892374
- PMCID: PMC5719467
- DOI: 10.1021/jacs.7b07223
Phase Segregation in Cs-, Rb- and K-Doped Mixed-Cation (MA)x(FA)1-xPbI3 Hybrid Perovskites from Solid-State NMR
Abstract
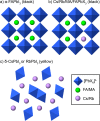
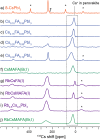
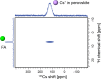
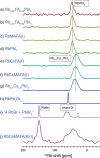
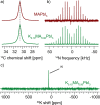
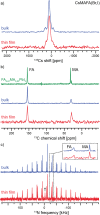
Hybrid (organic-inorganic) multication lead halide perovskites hold promise for a new generation of easily processable solar cells. Best performing compositions to date are multiple-cation solid alloys of formamidinium (FA), methylammonium (MA), cesium, and rubidium lead halides which provide power conversion efficiencies up to around 22%. Here, we elucidate the atomic-level nature of Cs and Rb incorporation into the perovskite lattice of FA-based materials. We use 133Cs, 87Rb, 39K, 13C, and 14N solid-state MAS NMR to probe microscopic composition of Cs-, Rb-, K-, MA-, and FA-containing phases in double-, triple-, and quadruple-cation lead halides in bulk and in a thin film. Contrary to previous reports, we have found no proof of Rb or K incorporation into the 3D perovskite lattice in these systems. We also show that the structure of bulk mechanochemical perovskites bears close resemblance to that of thin films, making them a good benchmark for structural studies. These findings provide fundamental understanding of previously reported excellent photovoltaic parameters in these systems and their superior stability.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Era M.; Hattori T.; Taira T.; Tsutsui T. Chem. Mater. 1997, 9 (1), 8–10. 10.1021/cm960434m. - DOI
-
- Kitazawa N.; Enomoto K.; Aono M.; Watanabe Y. J. Mater. Sci. 2004, 39 (2), 749–751. 10.1023/B:JMSC.0000011549.46602.d3. - DOI
-
- Liang K. N.; Mitzi D. B.; Prikas M. T. Chem. Mater. 1998, 10 (1), 403–411. 10.1021/cm970568f. - DOI
-
- Pradeesh K.; Baumberg J. J.; Prakash G. V. Appl. Phys. Lett. 2009, 95 (3), 033309–033311. 10.1063/1.3186639. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

