The equilibrium between antagonistic signaling pathways determines the number of synapses in Drosophila
- PMID: 28892511
- PMCID: PMC5593197
- DOI: 10.1371/journal.pone.0184238
The equilibrium between antagonistic signaling pathways determines the number of synapses in Drosophila
Abstract
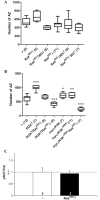
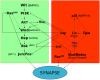
The number of synapses is a major determinant of behavior and many neural diseases exhibit deviations in that number. However, how signaling pathways control this number is still poorly understood. Using the Drosophila larval neuromuscular junction, we show here a PI3K-dependent pathway for synaptogenesis which is functionally connected with other previously known elements including the Wit receptor, its ligand Gbb, and the MAPkinases cascade. Based on epistasis assays, we determined the functional hierarchy within the pathway. Wit seems to trigger signaling through PI3K, and Ras85D also contributes to the initiation of synaptogenesis. However, contrary to other signaling pathways, PI3K does not require Ras85D binding in the context of synaptogenesis. In addition to the MAPK cascade, Bsk/JNK undergoes regulation by Puc and Ras85D which results in a narrow range of activity of this kinase to determine normalcy of synapse number. The transcriptional readout of the synaptogenesis pathway involves the Fos/Jun complex and the repressor Cic. In addition, we identified an antagonistic pathway that uses the transcription factors Mad and Medea and the microRNA bantam to down-regulate key elements of the pro-synaptogenesis pathway. Like its counterpart, the anti-synaptogenesis signaling uses small GTPases and MAPKs including Ras64B, Ras-like-a, p38a and Licorne. Bantam downregulates the pro-synaptogenesis factors PI3K, Hiw, Ras85D and Bsk, but not AKT. AKT, however, can suppress Mad which, in conjunction with the reported suppression of Mad by Hiw, closes the mutual regulation between both pathways. Thus, the number of synapses seems to result from the balanced output from these two pathways.
Conflict of interest statement
Figures




Similar articles
-
Synaptic and genomic responses to JNK and AP-1 signaling in Drosophila neurons.BMC Neurosci. 2005 Jun 2;6:39. doi: 10.1186/1471-2202-6-39. BMC Neurosci. 2005. PMID: 15932641 Free PMC article.
-
Morphogens and synaptogenesis in Drosophila.J Neurobiol. 2005 Sep 15;64(4):417-34. doi: 10.1002/neu.20165. J Neurobiol. 2005. PMID: 16041756 Review.
-
Age-independent synaptogenesis by phosphoinositide 3 kinase.J Neurosci. 2006 Oct 4;26(40):10199-208. doi: 10.1523/JNEUROSCI.1223-06.2006. J Neurosci. 2006. PMID: 17021175 Free PMC article.
-
Phosphoinositide-3-kinase activation controls synaptogenesis and spinogenesis in hippocampal neurons.J Neurosci. 2011 Feb 23;31(8):2721-33. doi: 10.1523/JNEUROSCI.4477-10.2011. J Neurosci. 2011. PMID: 21414895 Free PMC article.
-
Synaptic development: insights from Drosophila.Curr Opin Neurobiol. 2007 Feb;17(1):35-42. doi: 10.1016/j.conb.2007.01.001. Epub 2007 Jan 16. Curr Opin Neurobiol. 2007. PMID: 17229568 Review.
Cited by
-
Population genomics provides insights into the genetic diversity and adaptation of the Pieris rapae in China.PLoS One. 2023 Nov 16;18(11):e0294521. doi: 10.1371/journal.pone.0294521. eCollection 2023. PLoS One. 2023. PMID: 37972203 Free PMC article.
-
Insulin signaling mediates neurodegeneration in glioma.Life Sci Alliance. 2021 Feb 1;4(3):e202000693. doi: 10.26508/lsa.202000693. Print 2021 Mar. Life Sci Alliance. 2021. PMID: 33526430 Free PMC article.
-
A novel injury paradigm in the central nervous system of adult Drosophila: molecular, cellular and functional aspects.Dis Model Mech. 2021 May 1;14(5):dmm044669. doi: 10.1242/dmm.044669. Epub 2021 Jun 1. Dis Model Mech. 2021. PMID: 34061177 Free PMC article.
-
Alignment between glioblastoma internal clock and environmental cues ameliorates survival in Drosophila.Commun Biol. 2022 Jun 30;5(1):644. doi: 10.1038/s42003-022-03600-9. Commun Biol. 2022. PMID: 35773327 Free PMC article.
-
Multiple genetic loci affect place learning and memory performance in Drosophila melanogaster.Genes Brain Behav. 2019 Sep;18(7):e12581. doi: 10.1111/gbb.12581. Epub 2019 May 31. Genes Brain Behav. 2019. PMID: 31095869 Free PMC article.
References
-
- De Roo M, Klauser P, Muller D. LTP promotes a selective long-term stabilization and clustering of dendritic spines. PLoS biology. 2008;6(9):e219 Epub 2008/09/16. doi: 10.1371/journal.pbio.0060219 - DOI - PMC - PubMed
-
- Rasse TM, Fouquet W, Schmid A, Kittel RJ, Mertel S, Sigrist CB, et al. Glutamate receptor dynamics organizing synapse formation in vivo. Nature neuroscience. 2005;8(7):898–905. Epub 2005/09/02. - PubMed
-
- Gan WB, Kwon E, Feng G, Sanes JR, Lichtman JW. Synaptic dynamism measured over minutes to months: age-dependent decline in an autonomic ganglion. Nature neuroscience. 2003;6(9):956–60. Epub 2003/08/20. doi: 10.1038/nn1115 - DOI - PubMed
-
- Middei S, Ammassari-Teule M, Marie H. Synaptic plasticity under learning challenge. Neurobiology of learning and memory. 2014;115:108–15. Epub 2014/08/19. doi: 10.1016/j.nlm.2014.08.001 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

