The ASK1 inhibitor selonsertib in patients with nonalcoholic steatohepatitis: A randomized, phase 2 trial
- PMID: 28892558
- PMCID: PMC5814892
- DOI: 10.1002/hep.29514
The ASK1 inhibitor selonsertib in patients with nonalcoholic steatohepatitis: A randomized, phase 2 trial
Erratum in
-
Correction.Hepatology. 2018 May;67(5):2063. doi: 10.1002/hep.29875. Epub 2018 Mar 26. Hepatology. 2018. PMID: 29669401 Free PMC article. No abstract available.
Abstract
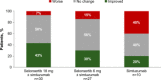
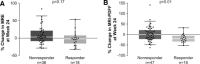
Inhibition of apoptosis signal-regulating kinase 1, a serine/threonine kinase, leads to improvement in inflammation and fibrosis in animal models of nonalcoholic steatohepatitis. We evaluated the safety and efficacy of selonsertib, a selective inhibitor of apoptosis signal-regulating kinase 1, alone or in combination with simtuzumab, in patients with nonalcoholic steatohepatitis and stage 2 or 3 liver fibrosis. In this multicenter phase 2 trial, 72 patients were randomized to receive 24 weeks of open-label treatment with either 6 or 18 mg of selonsertib orally once daily with or without once-weekly injections of 125 mg of simtuzumab or simtuzumab alone. The effect of treatment was assessed by paired pretreatment and posttreatment liver biopsies, magnetic resonance elastography, magnetic resonance imaging-estimated proton density fat fraction, quantitative collagen content, and noninvasive markers of liver injury. Due to the lack of effect of simtuzumab on histology or selonsertib pharmacokinetics, selonsertib groups with and without simtuzumab were pooled. After 24 weeks of treatment, the proportion of patients with a one or more stage reduction in fibrosis in the 18-mg selonsertib group was 13 of 30 (43%; 95% confidence interval, 26-63); in the 6-mg selonsertib group, 8 of 27 (30%; 95% confidence interval, 14-50); and in the simtuzumab-alone group, 2 of 10 (20%; 95% confidence interval, 3-56). Improvement in fibrosis was associated with reductions in liver stiffness on magnetic resonance elastography, collagen content and lobular inflammation on liver biopsy, as well as improvements in serum biomarkers of apoptosis and necrosis. There were no significant differences in adverse events between the treatment groups. Conclusion: These findings suggest that selonsertib may reduce liver fibrosis in patients with nonalcoholic steatohepatitis and stage 2-3 fibrosis. (Hepatology 2018;67:549-559).
© 2017 The Authors. Hepatology published by Wiley Periodicals, Inc., on behalf of the American Association for the Study of Liver Diseases.
Figures

Comment in
-
NAFLD: Early promise for ASK1 inhibition in NASH.Nat Rev Gastroenterol Hepatol. 2017 Nov;14(11):631. doi: 10.1038/nrgastro.2017.144. Epub 2017 Oct 11. Nat Rev Gastroenterol Hepatol. 2017. PMID: 29018270 No abstract available.
References
-
- Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005;41:1313‐1321. - PubMed
-
- Younossi ZM, Stepanova M, Rafiq N, Makhlouf H, Younoszai Z, Agrawal R, et al. Pathologic criteria for nonalcoholic steatohepatitis: interprotocol agreement and ability to predict liver‐related mortality. Hepatology 2011;53:1874‐1882. - PubMed
-
- Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The diagnosis and management of non‐alcoholic fatty liver disease: practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 2012;55:2005‐2023. - PubMed
-
- Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non‐alcoholic fatty liver disease and non‐alcoholic steatohepatitis in adults. Aliment Pharmacol Ther 2011;34:274‐285. - PubMed
-
- Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease—meta‐analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73‐84. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

