Evaluation design of Urban Health Centres Europe (UHCE): preventive integrated health and social care for community-dwelling older persons in five European cities
- PMID: 28893178
- PMCID: PMC5594491
- DOI: 10.1186/s12877-017-0606-1
Evaluation design of Urban Health Centres Europe (UHCE): preventive integrated health and social care for community-dwelling older persons in five European cities
Abstract
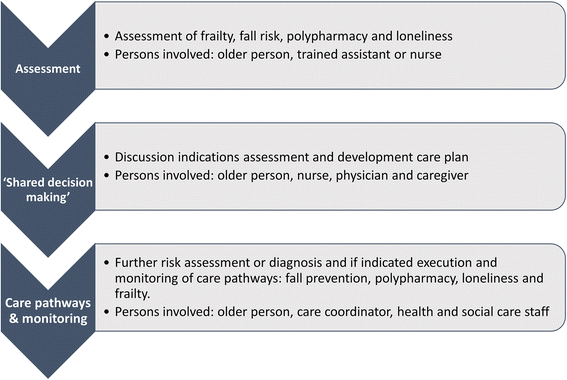
Background: Older persons often have interacting physical and social problems and complex care needs. An integrated care approach in the local context with collaborations between community-, social-, and health-focused organisations can contribute to the promotion of independent living and quality of life. In the Urban Health Centres Europe (UHCE) project, five European cities (Greater Manchester, United Kingdom; Pallini (in Greater Athens Area), Greece; Rijeka, Croatia; Rotterdam, the Netherlands; and Valencia, Spain) develop and implement a care template that integrates health and social care and includes a preventive approach. The UHCE project includes an effect and process evaluation.
Methods: In a one-year pre-post controlled trial, in each city 250 participants aged 75+ years are recruited to receive the UHCE approach and are compared with 250 participants who receive 'care as usual'. Benefits of UHCE approach in terms of healthy life styles, fall risk, appropriate medication use, loneliness level and frailty, and in terms of level of independence and health-related quality of life and health care use are assessed. A multilevel modeling approach is used for the analyses. The process evaluation is used to provide insight into the reach of the target population, the extent to which elements of the UHCE approach are executed as planned and the satisfaction of the participants.
Discussion: The UHCE project will provide new insight into the feasibility and effectiveness of an integrated care approach for older persons in different European settings.
Trial registration: ISRCTN registry number is ISRCTN52788952 . Date of registration is 13/03/2017.
Keywords: Frailty; Integrated health and social care; Older citizens; Prevention; Primary care; Specific pre-post controlled clinical trial.
Conflict of interest statement
Ethics approval and consent to participate
Ethical committee procedures have been followed in all cities and institutions involved, and approval has been provided. The names of the review board and the approval references are: Manchester, United Kingdom: NRES Committee West Midlands - Coventry & Warwickshire; 06–03-2015; 15/WM/0080; NRES Committee South Central – Berkshire B; 29–20-2014; 14/SC/1349; Pallini, Greece: The Ethics and Scientific board - Latriko Palaiou Falirou Hospital; 04/03/2015; 20,150,304–01; Rijeka, Croatia: The Ethical Committee - Faculty of Medicine University of Rijeka; 07–04-2014; 2170–24–01-14-02; Rotterdam, The Netherlands: Medische Ethische Toetsings Commissie (METC) – Erasmus MC Rotterdam; 08/01/2015; MEC-2014-661; Valencia, Spain: Comisión de Investigación - Consorcio Hospital General Universitario de Valencia. 29/01/2015; CICHGUV-2015-01-29. Written consent is obtained from all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
-
- Eurostat 2016, Population structure and ageing. http://epp.eurostat.ec.europa.eu/statistics_explained/index.php/Populati....
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources