Trial design and rationale for APOLLO, a Phase 3, placebo-controlled study of patisiran in patients with hereditary ATTR amyloidosis with polyneuropathy
- PMID: 28893208
- PMCID: PMC5594468
- DOI: 10.1186/s12883-017-0948-5
Trial design and rationale for APOLLO, a Phase 3, placebo-controlled study of patisiran in patients with hereditary ATTR amyloidosis with polyneuropathy
Abstract
Background: Patisiran is an investigational RNA interference (RNAi) therapeutic in development for the treatment of hereditary ATTR (hATTR) amyloidosis, a progressive disease associated with significant disability, morbidity, and mortality.
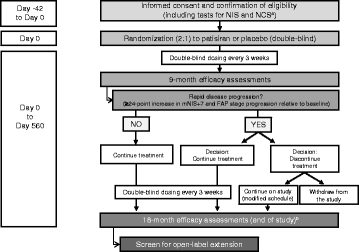
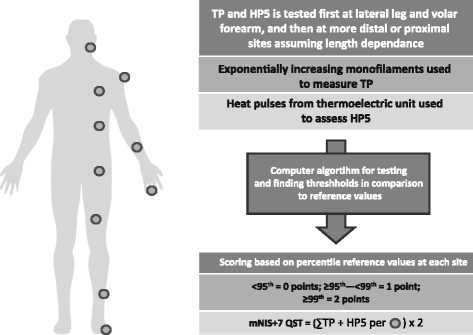
Methods: Here we describe the rationale and design of the Phase 3 APOLLO study, a randomized, double-blind, placebo-controlled, global study to evaluate the efficacy and safety of patisiran in patients with hATTR amyloidosis with polyneuropathy. Eligible patients are 18-85 years old with hATTR amyloidosis, investigator-estimated survival of ≥2 years, Neuropathy Impairment Score (NIS) of 5-130, and polyneuropathy disability score ≤IIIb. Patients are randomized 2:1 to receive either intravenous patisiran 0.3 mg/kg or placebo once every 3 weeks. The primary objective is to determine the efficacy of patisiran at 18 months based on the difference in the change in modified NIS+7 (a composite measure of motor strength, sensation, reflexes, nerve conduction, and autonomic function) between the patisiran and placebo groups. Secondary objectives are to evaluate the effect of patisiran on Norfolk-Diabetic Neuropathy quality of life questionnaire score, nutritional status (as evaluated by modified body mass index), motor function (as measured by NIS-weakness and timed 10-m walk test), and autonomic symptoms (as measured by the Composite Autonomic Symptom Score-31 questionnaire). Exploratory objectives include assessment of cardiac function and pathologic evaluation to assess nerve fiber innervation and amyloid burden. Safety of patisiran will be assessed throughout the study.
Discussion: APOLLO represents the largest randomized, Phase 3 study to date in patients with hATTR amyloidosis, with endpoints that capture the multisystemic nature of this disease.
Trial registration: This trial is registered at clinicaltrials.gov ( NCT01960348 ); October 9, 2013.
Keywords: APOLLO; Methods; Patisiran; Polyneuropathy; RNA interference; hATTR amyloidosis; mNIS+7.
Conflict of interest statement
Correspondence information for trial sponsor
Alnylam Pharmaceuticals Cambridge, 300 Third Street, 3rd Floor, Cambridge, MA 02142, USA. Tel: 00 1 617 551 8200; Fax: 00 1 617 551 8101.
Ethics approval and consent to participate
This study is conducted according to the guidelines of the International Conference on Harmonisation, the World Health Organization’s Declaration of Helsinki, and the Health Insurance Portability and Accountability Act of 1996. Written informed consent is obtained from all patients who participate in the study, prior to assessment of eligibility. The study protocol was approved by the local Institutional Review Boards and Ethics Committees, and all subsequent protocol amendments underwent the same approval procedure.
Consent for publication
Not applicable.
Competing interests
Adams: Research funding and consultancy fees from Alnylam Pharmaceuticals, and personal symposium fees from Pfizer Inc. Suhr: Personal fees and non-financial support from Alnylam Pharmaceuticals, and personal fees from Prothena Pharmaceuticals and Pfizer Inc. Dyck: Compensation for training and quality assurance associated with clinical trials from Alnylam Pharmaceuticals and IONIS Pharmaceuticals; honoraria from Diabetes journal as Associate Editor. Litchy: Compensation for travel and training of investigators from Alnylam Pharmaceuticals and IONIS Pharmaceuticals. Leahy: Employment by Alnylam Pharmaceuticals. Chen: Employment by Alnylam Pharmaceuticals. Gollob: Employment by Alnylam Pharmaceuticals. Coelho: Compensation for training from Pfizer Inc. and Alnylam Pharmaceuticals, and compensation for travel from Pfizer Inc., Alnylam Pharmaceuticals, and IONIS Pharmaceuticals.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Coelho T, Maurer MS, Suhr OB. THAOS - The Transthyretin Amyloidosis Outcomes Survey: initial report on clinical manifestations in patients with hereditary and wild-type transthyretin amyloidosis. Curr Med Res Opin. 2013;29(1):63–76. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

