Development of an anti-HIV vaccine eliciting broadly neutralizing antibodies
- PMID: 28893278
- PMCID: PMC5594608
- DOI: 10.1186/s12981-017-0178-3
Development of an anti-HIV vaccine eliciting broadly neutralizing antibodies
Abstract
The extreme HIV diversity posts a great challenge on development of an effective anti-HIV vaccine. To solve this problem, it is crucial to discover an appropriate immunogens and strategies that are able to prevent the transmission of the diverse viruses that are circulating in the world. Even though there have been a number of broadly neutralizing anti-HIV antibodies (bNAbs) been discovered in recent years, induction of such antibodies to date has only been observed in HIV-1 infection. Here, in this mini review, we review the progress in development of HIV vaccine in eliciting broad immune response, especially production of bNAbs, discuss possible strategies, such as polyvalent sequential vaccination, that facilitates B cell maturation leading to bNAb response.
Keywords: B cell maturation; Broadly neutralizing antibody; Diversity; HIV-1; Polyvalent vaccine.
Figures
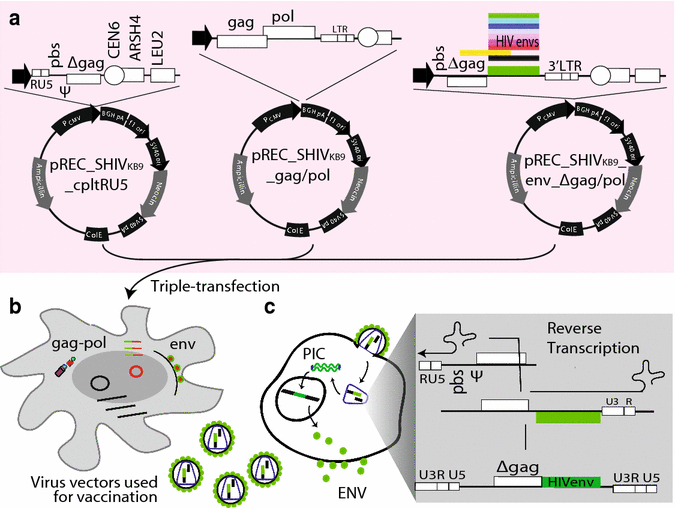
References
-
- Cho MW, Kim YB, Lee MK, Gupta KC, Ross W, Plishka R, et al. Polyvalent envelope glycoprotein vaccine elicits a broader neutralizing antibody response but is unable to provide sterilizing protection against heterologous simian/human immunodeficiency virus infection in pigtailed macaques. J Virol. 2001;75:2224–2234. doi: 10.1128/JVI.75.5.2224-2234.2001. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

