Prevalence and trends in transmitted and acquired antiretroviral drug resistance, Washington, DC, 1999-2014
- PMID: 28893321
- PMCID: PMC5594524
- DOI: 10.1186/s13104-017-2764-9
Prevalence and trends in transmitted and acquired antiretroviral drug resistance, Washington, DC, 1999-2014
Abstract
Background: Drug resistance limits options for antiretroviral therapy (ART) and results in poorer health outcomes among HIV-infected persons. We sought to characterize resistance patterns and to identify predictors of resistance in Washington, DC.
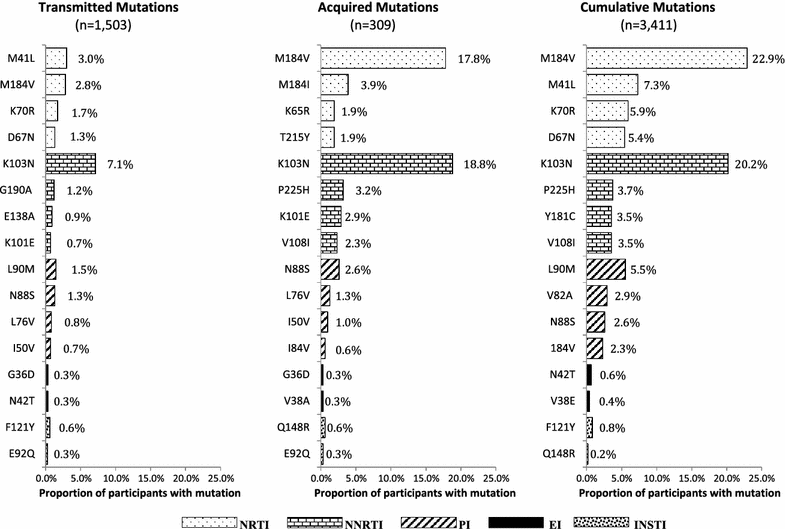
Methods: We analyzed resistance in the DC Cohort, a longitudinal study of HIV-infected persons in care in Washington, DC. We measured cumulative drug resistance (CDR) among participants with any genotype between 1999 and 2014 (n = 3411), transmitted drug resistance (TDR) in ART-naïve persons (n = 1503), and acquired drug resistance (ADR) in persons with genotypes before and after ART initiation (n = 309). Using logistic regression, we assessed associations between patient characteristics and transmitted resistance to any antiretroviral.
Results: Prevalence of TDR was 20.5%, of ADR 40.5%, and of CDR 45.1% in the respective analysis groups. From 2004 to 2013, TDR prevalence decreased for nucleoside and nucleotide analogue reverse transcriptase inhibitors (15.0 to 5.5%; p = 0.0003) and increased for integrase strand transfer inhibitors (INSTIs) (0.0-1.4%; p = 0.04). In multivariable analysis, TDR was not associated with age, race/ethnicity, HIV risk group, or years from HIV diagnosis.
Conclusions: In this urban cohort of HIV-infected persons, almost half of participants tested had evidence of CDR; and resistance to INSTIs was increasing. If this trend continues, inclusion of the integrase-encoding region in baseline genotype testing should be strongly considered.
Keywords: Acquired drug resistance; Antiretroviral therapy; Cumulative drug resistance; DC; Drug resistance; HIV; Prevalence; Transmitted drug resistance; Washington.
Figures


Similar articles
-
Geographic and temporal trends of transmitted HIV-1 drug resistance among antiretroviral-naïve subjects screening for two clinical trials in North America and Western Europe.HIV Clin Trials. 2009 Mar-Apr;10(2):94-103. doi: 10.1310/hct1002-94. HIV Clin Trials. 2009. PMID: 19487179
-
Antiretroviral drug resistance among antiretroviral-naïve and treatment experienced patients infected with HIV in Iran.J Med Virol. 2014 Jul;86(7):1093-8. doi: 10.1002/jmv.23898. Epub 2014 Apr 17. J Med Virol. 2014. PMID: 24740443
-
Trends in drug resistance prevalence in HIV-1-infected children in Madrid: 1993 to 2010 analysis.Pediatr Infect Dis J. 2012 Nov;31(11):e213-21. doi: 10.1097/INF.0b013e3182678c7c. Pediatr Infect Dis J. 2012. PMID: 22785049
-
Antiretroviral treatment sequencing strategies to overcome HIV type 1 drug resistance in adolescents and adults in low-middle-income countries.J Infect Dis. 2013 Jun 15;207 Suppl 2:S63-9. doi: 10.1093/infdis/jit109. J Infect Dis. 2013. PMID: 23687291 Review.
-
Study of the impact of HIV genotypic drug resistance testing on therapy efficacy.Verh K Acad Geneeskd Belg. 2001;63(5):447-73. Verh K Acad Geneeskd Belg. 2001. PMID: 11813503 Review.
Cited by
-
HIV Drug Resistance in Children and Adolescents: Always a Challenge?Curr Epidemiol Rep. 2021;8(3):97-107. doi: 10.1007/s40471-021-00268-3. Epub 2021 Mar 18. Curr Epidemiol Rep. 2021. PMID: 33758743 Free PMC article. Review.
-
Statewide Longitudinal Trends in Transmitted HIV-1 Drug Resistance in Rhode Island, USA.Open Forum Infect Dis. 2021 Dec 7;9(1):ofab587. doi: 10.1093/ofid/ofab587. eCollection 2022 Jan. Open Forum Infect Dis. 2021. PMID: 34988256 Free PMC article.
-
HIV-1-Transmitted Drug Resistance and Transmission Clusters in Newly Diagnosed Patients in Portugal Between 2014 and 2019.Front Microbiol. 2022 Apr 25;13:823208. doi: 10.3389/fmicb.2022.823208. eCollection 2022. Front Microbiol. 2022. PMID: 35558119 Free PMC article.
-
Real-World Adherence to Antiretroviral Therapy Among HIV-1 Patients Across the United States.Adv Ther. 2021 Sep;38(9):4961-4974. doi: 10.1007/s12325-021-01883-8. Epub 2021 Aug 14. Adv Ther. 2021. PMID: 34390465 Free PMC article.
-
Acquired Human Immunodeficiency Virus Type 1 Drug Resistance in Rhode Island, USA, 2004-2021.J Infect Dis. 2024 Dec 16;230(6):1422-1433. doi: 10.1093/infdis/jiae344. J Infect Dis. 2024. PMID: 39041648 Free PMC article.
References
-
- D’Aquila RT, Johnson VA, Welles SL, Japour AJ, Kuritzkes DR, DeGruttola V, et al. Zidovudine resistance and HIV-1 disease progression during antiretroviral therapy. AIDS Clinical Trials Group Protocol 116B/117 Team and the Virology Committee Resistance Working Group. Ann Intern Med. 1995;122:401–408. doi: 10.7326/0003-4819-122-6-199503150-00001. - DOI - PubMed
-
- Havlir DV, Marschner IC, Hirsch MS, Collier AC, Tebas P, Bassett RL, et al. Maintenance antiretroviral therapies in HIV infected patients with undetectable plasma HIV RNA after triple-drug therapy. AIDS Clinical Trials Group Study 343 Team. N Engl J Med. 1998;339:1261–1268. doi: 10.1056/NEJM199810293391801. - DOI - PubMed
-
- Rey D, Hughes M, Pi JT, Winters M, Merigan TC, Katzenstein DA. HIV-1 reverse transcriptase codon 215 mutation in plasma RNA: immunologic and virologic responses to zidovudine. The AIDS Clinical Trials Group Study 175 Virology Team. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;17:203–208. doi: 10.1097/00042560-199803010-00003. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

