Hepatic Ischemia/Reperfusion: Mechanisms of Tissue Injury, Repair, and Regeneration
- PMID: 28893351
- PMCID: PMC5885149
- DOI: 10.3727/105221617X15042750874156
Hepatic Ischemia/Reperfusion: Mechanisms of Tissue Injury, Repair, and Regeneration
Abstract
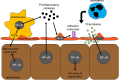
Hepatic ischemia/reperfusion (I/R) injury is a major complication of liver surgery, including liver resection, liver transplantation, and trauma surgery. Much has been learned about the inflammatory injury response induced by I/R, including the cascade of proinflammatory mediators and recruitment of activated leukocytes. In this review, we discuss the complex network of events that culminate in liver injury after I/R, including cellular, protein, and molecular mechanisms. In addition, we address the known endogenous regulatory mediators that function to maintain homeostasis and resolve injury. Finally, we cover more recent insights into how the liver repairs and regenerates after I/R injury, a setting in which physical mass remains unchanged, but functional liver mass is greatly reduced. In this regard, we focus on recent work highlighting a novel role of CXC chemokines as important regulators of hepatocyte proliferation and liver regeneration after I/R injury.
Figures


References
-
- Huguet C, Gavelli A, Bona S. Hepatic resection with ischemia of the liver exceeding one hour. J Am Coll Surg. 1994;178(5):454–8. - PubMed
-
- Lemasters JJ, Thurman RG. Reperfusion injury after liver preservation for transplantation. Annu Rev Pharmacol Toxicol. 1997;37:327–38. - PubMed
-
- Kim YI. Ischemia-reperfusion injury of the human liver during hepatic resection. J Hepatobiliary Pancreat Surg. 2003;10(3):195–9. - PubMed
-
- Jaeschke H, Bautista AP, Spolarics Z, Spitzer JJ. Superoxide generation by Kupffer cells and priming of neutrophils during reperfusion after hepatic ischemia. Free Radic Res Commun. 1991;15(5):277–84. - PubMed
-
- Jaeschke H, Farhood A. Neutrophil and Kupffer cell-induced oxidant stress and ischemia-reperfusion injury in rat liver. Am J Physiol. 1991;260(3 Pt 1):G355–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
