Induced sensorimotor cortex plasticity remediates chronic treatment-resistant visual neglect
- PMID: 28893377
- PMCID: PMC5595432
- DOI: 10.7554/eLife.26602
Induced sensorimotor cortex plasticity remediates chronic treatment-resistant visual neglect
Abstract
Right brain injury causes visual neglect - lost awareness of left space. During prism adaptation therapy, patients adapt to a rightward optical shift by recalibrating right arm movements leftward. This can improve left neglect, but the benefit of a single session is transient (~1 day). Here we show that tonic disinhibition of left motor cortex during prism adaptation enhances consolidation, stabilizing both sensorimotor and cognitive prism after-effects. In three longitudinal patient case series, just 20 min of combined stimulation/adaptation caused persistent cognitive after-effects (neglect improvement) that lasted throughout follow-up (18-46 days). Moreover, adaptation without stimulation was ineffective. Thus stimulation reversed treatment resistance in chronic visual neglect. These findings challenge consensus that because the left hemisphere in neglect is pathologically over-excited it ought to be suppressed. Excitation of left sensorimotor circuits, during an adaptive cognitive state, can unmask latent plastic potential that durably improves resistant visual attention deficits after brain injury.
Keywords: human; motor memory consolidation; neglect rehabilitation; neuroscience; prism adaptation.
Conflict of interest statement
Reviewing editor,
No competing interests declared.
Figures


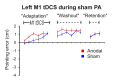

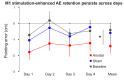

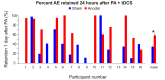



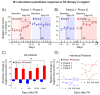

References
-
- Buch ER, Santarnecchi E, Antal A, Born J, Celnik PA, Classen J, Gerloff C, Hallett M, Hummel FC, Nitsche MA, Pascual-Leone A, Paulus WJ, Reis J, Robertson EM, Rothwell JC, Sandrini M, Schambra HM, Wassermann EM, Ziemann U, Cohen LG. Effects of tDCS on motor learning and memory formation: A consensus and critical position paper. Clinical Neurophysiology. 2017;128:589–603. doi: 10.1016/j.clinph.2017.01.004. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

