Localization of spontaneous bursting neuronal activity in the preterm human brain with simultaneous EEG-fMRI
- PMID: 28893378
- PMCID: PMC5595428
- DOI: 10.7554/eLife.27814
Localization of spontaneous bursting neuronal activity in the preterm human brain with simultaneous EEG-fMRI
Abstract
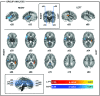
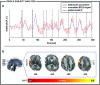
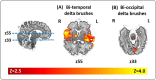
Electroencephalographic recordings from the developing human brain are characterized by spontaneous neuronal bursts, the most common of which is the delta brush. Although similar events in animal models are known to occur in areas of immature cortex and drive their development, their origin in humans has not yet been identified. Here, we use simultaneous EEG-fMRI to localise the source of delta brush events in 10 preterm infants aged 32-36 postmenstrual weeks. The most frequent patterns were left and right posterior-temporal delta brushes which were associated in the left hemisphere with ipsilateral BOLD activation in the insula only; and in the right hemisphere in both the insular and temporal cortices. This direct measure of neural and hemodynamic activity shows that the insula, one of the most densely connected hubs in the developing cortex, is a major source of the transient bursting events that are critical for brain maturation.
Keywords: EEG; brain development; fMRI; human; human biology; medicine; neonate; neuroscience; prematurity; spontaneous neuronal activity.
Conflict of interest statement
No competing interests declared.
Figures


Comment in
-
Structured Spontaneity: Building Circuits in the Human Prenatal Brain.Trends Neurosci. 2018 Jan;41(1):1-3. doi: 10.1016/j.tins.2017.11.004. Epub 2017 Dec 7. Trends Neurosci. 2018. PMID: 29224852 Free PMC article.
References
-
- American Clinical Neurophysiology Society Critical Care Monitoring Committee. Tsuchida TN, Wusthoff CJ, Shellhaas RA, Abend NS, Hahn CD, Sullivan JE, Nguyen S, Weinstein S, Scher MS, Riviello JJ, Clancy RR. American clinical neurophysiology society standardized EEG terminology and categorization for the description of continuous EEG monitoring in neonates: report of the American Clinical Neurophysiology Society critical care monitoring committee. Journal of Clinical Neurophysiology : Official Publication of the American Electroencephalographic Society. 2013;30:161–173. doi: 10.1097/WNP.0b013e3182872b24. - DOI - PubMed
-
- André M, Lamblin MD, d'Allest AM, Curzi-Dascalova L, Moussalli-Salefranque F, S Nguyen The T, Vecchierini-Blineau MF, Wallois F, Walls-Esquivel E, Plouin P. Electroencephalography in premature and full-term infants. Developmental features and glossary. Neurophysiologie Clinique/Clinical Neurophysiology. 2010;40:59–124. doi: 10.1016/j.neucli.2010.02.002. - DOI - PubMed
-
- Arichi T, Moraux A, Melendez A, Doria V, Groppo M, Merchant N, Combs S, Burdet E, Larkman DJ, Counsell SJ, Beckmann CF, Edwards AD. Somatosensory cortical activation identified by functional MRI in preterm and term infants. NeuroImage. 2010;49:2063–2071. doi: 10.1016/j.neuroimage.2009.10.038. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

