Tyrphostin RG14620 selectively reverses ABCG2-mediated multidrug resistance in cancer cell lines
- PMID: 28893612
- PMCID: PMC5634936
- DOI: 10.1016/j.canlet.2017.08.035
Tyrphostin RG14620 selectively reverses ABCG2-mediated multidrug resistance in cancer cell lines
Abstract
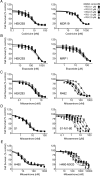
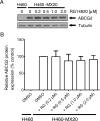
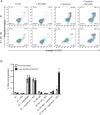
The multidrug resistance (MDR) phenotype associated with the overexpression of ATP-binding cassette (ABC) drug transporters ABCB1, ABCC1 and ABCG2 is a major obstacle in cancer chemotherapy. Numerous epidermal growth factor receptor (EGFR) inhibitors have previously been shown capable of reversing MDR in ABCG2-overexpressing cancer cells. However, most of them are not transporter-specific due to the substantial overlapping substrate specificity among the transporters. In this study, we investigated the interaction between ABCG2 and tyrphostin RG14620, an EGFR inhibitor of the tyrphostin family, in multidrug-resistant cancer cell lines. We found that at nontoxic concentrations, tyrphostin RG14620 enhances drug-induced apoptosis and restores chemosensitivity to ABCG2-overexpressing multidrug-resistant cancer cells. More importantly, tyrphostin RG14620 is selective to ABCG2 relative to ABCB1 and ABCC1. Our findings were further supported by biochemical assays demonstrating that tyrphostin RG14620 stimulates ATP hydrolysis and inhibits photoaffinity labeling of ABCG2 with IAAP, and by a docking analysis of tyrphostin RG14620 in the drug-binding pocket of this transporter. Taken together, our findings indicate that tyrphostin RG14620 is a potent and selective modulator of ABCG2 that may be useful to overcome chemoresistance in patients with drug-resistant tumors.
Keywords: ABC transporter; ABCG2; EGFR inhibitor; Multidrug resistance; Tyrphostin RG14620.
Copyright © 2017 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Ahmad SF, Ansari MA, Nadeem A, Zoheir KM, Bakheet SA, Al-Shabanah OA, Al Rikabi AC, Attia SM. The tyrosine kinase inhibitor tyrphostin AG126 reduces activation of inflammatory cells and increases Foxp3+ regulatory T cells during pathogenesis of rheumatoid arthritis. Molecular immunology. 2016;78:65–78. - PubMed
-
- Allen JD, Schinkel AH. Multidrug resistance and pharmacological protection mediated by the breast cancer resistance protein (BCRP/ABCG2) Mol Cancer Ther. 2002;1:427–434. - PubMed
-
- Ambudkar SV. Drug-stimulatable ATPase activity in crude membranes of human MDR1-transfected mammalian cells. Methods Enzymol. 1998;292:504–514. - PubMed
-
- Ambudkar SV, Dey S, Hrycyna CA, Ramachandra M, Pastan I, Gottesman MM. Biochemical, cellular, and pharmacological aspects of the multidrug transporter. Annual review of pharmacology and toxicology. 1999;39:361–398. - PubMed
-
- Ambudkar SV, Kimchi-Sarfaty C, Sauna ZE, Gottesman MM. P-glycoprotein: from genomics to mechanism. Oncogene. 2003;22:7468–7485. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

