Effectiveness of expanding annual mass azithromycin distribution treatment coverage for trachoma in Niger: a cluster randomised trial
- PMID: 28893761
- PMCID: PMC5845832
- DOI: 10.1136/bjophthalmol-2017-310916
Effectiveness of expanding annual mass azithromycin distribution treatment coverage for trachoma in Niger: a cluster randomised trial
Abstract
Background/aims: The WHO recommends 3-5 years of annual mass azithromycin distribution with at least 80% treatment coverage to districts with active trachoma prevalence over 10% among children. Here, we assess the efficacy of expanding the coverage target to at least 90% for trachoma control in a mesoendemic region of Niger.
Methods: Twenty-four communities were randomised to a single day of azithromycin distribution with a coverage target of 80% of the community or up to 4 days of treatment, aiming for greater than 90% coverage. Distributions were annual and individuals above 6 months of age were treated. Children under 5 years of age were monitored for ocular chlamydia infection and active trachoma.
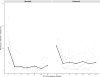
Results: At baseline, ocular chlamydia prevalence was 20.5% (95% CI 9.8% to 31.2%) in the standard coverage arm and 21.9% (95% CI 11.3% to 32.5%) in the enhanced coverage arm, which reduced to 4.6% (95% CI 0% to 9.5%, p=0.008) and 7.1% (95% CI 2.7% to 11.4%, p<0.001) at 36 months, respectively. There was no significant difference in 36-month ocular chlamydia prevalence between the two arms (p=0.21). There was no difference in the rate of decline in ocular chlamydia between the two arms in a repeated measures model (p=0.80).
Conclusions: For annual mass azithromycin distribution programme to an entire community, there may be no additional benefit of increasing antibiotic coverage above the WHO's 80% target.
Trial registration number: NCT00792922, post-results.
Keywords: Child Health (paediatrics); Clinical Trial; Conjunctiva; Infection; Public Health.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2018. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: None declared.
Figures



References
-
- World Health Organization. Report of the 17th Meeting of the WHO Alliance for the Global Elimination of Blinding Trachoma. Geneva: 2013. Nov, pp. 1–56.
-
- Melese M, Chidambaram JD, Alemayehu W, Lee DC, Yi EH, Cevallos V, et al. Feasibility of Eliminating Ocular Chlamydia trachomatis With Repeat Mass Antibiotic Treatments. JAMA. 2004;292:721–725. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
