Gemini Cationic Amphiphiles Control Biofilm Formation by Bacterial Vaginosis Pathogens
- PMID: 28893789
- PMCID: PMC5700334
- DOI: 10.1128/AAC.00650-17
Gemini Cationic Amphiphiles Control Biofilm Formation by Bacterial Vaginosis Pathogens
Abstract
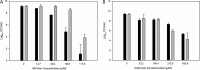
Antibiotic resistance and recurrence of bacterial vaginosis (BV), a polymicrobial infection, justify the need for novel antimicrobials to counteract microbial resistance to conventional antibiotics. Previously, two series of cationic amphiphiles (CAms) which self-assemble into supramolecular nanostructures with membrane-lytic properties were designed with hydrophilic head groups and nonpolar domains. The combination of CAms and commonly prescribed antibiotics is suggested as a promising strategy for targeting microorganisms that are resistant to conventional antibiotics. Activities of the CAms against Gardnerella vaginalis ATCC 14018, a representative BV pathogen, ranged from 1.1 to 24.4 μM. Interestingly, the tested healthy Lactobacillus species, especially Lactobacillus plantarum ATCC 39268, were significantly more tolerant of CAms than the selected pathogens. In addition, CAms prevented biofilm formation at concentrations which did not influence the normal growth ability of G. vaginalis ATCC 14018. Furthermore, the biofilm minimum bactericidal concentration (MBC-Bs) of CAms against G. vaginalis ATCC 14018 ranged from 58.8 to 425.6 μM, while much higher concentrations (≥850 μM) were required to produce ≥3-log reductions in the number of biofilm-associated lactobacilli. The conventional antibiotic metronidazole synergized strongly with all tested CAms against planktonic cells and biofilms of G. vaginalis ATCC 14018. The synergism between CAms and the tested conventional antibiotic may be considered a new, effective, and beneficial method of controlling biofilm-associated bacterial vaginosis.
Keywords: AMP mimics; Gardnerella vaginalis; antimicrobials; bacterial vaginosis; biofilm.
Copyright © 2017 American Society for Microbiology.
Figures
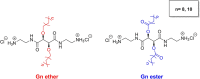




References
-
- Allsworth JE, Peipert JF. 2007. Prevalence of bacterial vaginosis: 2001-2004 National Health and Nutrition Examination Survey data. Obstet Gynecol 109:114–120. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

