Chemical Genetic Interaction Profiling Reveals Determinants of Intrinsic Antibiotic Resistance in Mycobacterium tuberculosis
- PMID: 28893793
- PMCID: PMC5700314
- DOI: 10.1128/AAC.01334-17
Chemical Genetic Interaction Profiling Reveals Determinants of Intrinsic Antibiotic Resistance in Mycobacterium tuberculosis
Abstract
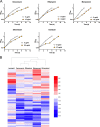
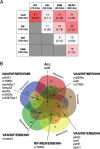
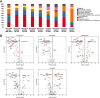
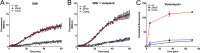
Chemotherapy for tuberculosis (TB) is lengthy and could benefit from synergistic adjuvant therapeutics that enhance current and novel drug regimens. To identify genetic determinants of intrinsic antibiotic susceptibility in Mycobacterium tuberculosis, we applied a chemical genetic interaction (CGI) profiling approach. We screened a saturated transposon mutant library and identified mutants that exhibit altered fitness in the presence of partially inhibitory concentrations of rifampin, ethambutol, isoniazid, vancomycin, and meropenem, antibiotics with diverse mechanisms of action. This screen identified the M. tuberculosis cell envelope to be a major determinant of antibiotic susceptibility but did not yield mutants whose increase in susceptibility was due to transposon insertions in genes encoding efflux pumps. Intrinsic antibiotic resistance determinants affecting resistance to multiple antibiotics included the peptidoglycan-arabinogalactan ligase Lcp1, the mycolic acid synthase MmaA4, the protein translocase SecA2, the mannosyltransferase PimE, the cell envelope-associated protease CaeA/Hip1, and FecB, a putative iron dicitrate-binding protein. Characterization of a deletion mutant confirmed FecB to be involved in the intrinsic resistance to every antibiotic analyzed. In contrast to its predicted function, FecB was dispensable for growth in low-iron medium and instead functioned as a critical mediator of envelope integrity.
Keywords: Mycobacterium tuberculosis; antibiotic resistance; cell envelope; mutational studies.
Copyright © 2017 American Society for Microbiology.
Figures


References
-
- World Health Organization. 2010. Guidelines for treatment of tuberculosis, 4th ed World Health Organization, Geneva, Switzerland: http://apps.who.int/iris/bitstream/10665/44165/1/9789241547833_eng.pdf.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

