Coordination between Differentially Regulated Circadian Clocks Generates Rhythmic Behavior
- PMID: 28893860
- PMCID: PMC6028074
- DOI: 10.1101/cshperspect.a033589
Coordination between Differentially Regulated Circadian Clocks Generates Rhythmic Behavior
Abstract
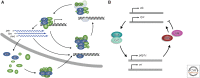
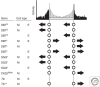
Specialized groups of neurons in the brain are key mediators of circadian rhythms, receiving daily environmental cues and communicating those signals to other tissues in the organism for entrainment and to organize circadian physiology. In Drosophila, the "circadian clock" is housed in seven neuronal clusters, which are defined by their expression of the main circadian proteins, Period, Timeless, Clock, and Cycle. These clusters are distributed across the fly brain and are thereby subject to the respective environments associated with their anatomical locations. While these core components are universally expressed in all neurons of the circadian network, additional regulatory proteins that act on these components are differentially expressed, giving rise to "local clocks" within the network that nonetheless converge to regulate coherent behavioral rhythms. In this review, we describe the communication between the neurons of the circadian network and the molecular differences within neurons of this network. We focus on differences in protein-expression patterns and discuss how such variation can impart functional differences in each local clock. Finally, we summarize our current understanding of how communication within the circadian network intersects with intracellular biochemical mechanisms to ultimately specify behavioral rhythms. We propose that additional efforts are required to identify regulatory mechanisms within each neuronal cluster to understand the molecular basis of circadian behavior.
Copyright © 2018 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures

References
-
- Akten B, Jauch E, Genova GK, Kim EY, Edery I, Raabe T, Jackson FR. 2003. A role for CK2 in the Drosophila circadian oscillator. Nat Neurosci 6: 251–257. - PubMed
-
- Allada R, White NE, So WV, Hall JC, Rosbash M. 1998. A mutant Drosophila homolog of mammalian Clock disrupts circadian rhythms and transcription of period and timeless. Cell 93: 791–804. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
