Human T-cell leukaemia virus type 1: parasitism and pathogenesis
- PMID: 28893939
- PMCID: PMC5597739
- DOI: 10.1098/rstb.2016.0272
Human T-cell leukaemia virus type 1: parasitism and pathogenesis
Abstract
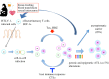
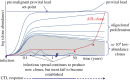
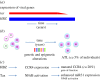
Human T-cell leukaemia virus type 1 (HTLV-1) causes not only adult T-cell leukaemia-lymphoma (ATL), but also inflammatory diseases including HTLV-1-associated myelopathy/tropical spastic paraparesis. HTLV-1 transmits primarily through cell-to-cell contact, and generates abundant infected cells in the host in order to survive and transmit to a new host. The resulting high proviral load is closely associated with the development of ATL and inflammatory diseases. To increase the number of infected cells, HTLV-1 changes the immunophenotype of infected cells, induces proliferation and inhibits apoptosis through the cooperative actions of two viral genes, tax and HTLV-1 bZIP factor (HBZ). As a result, infected cells survive, proliferate and infiltrate into the tissues, which is critical for transmission of the virus. Thus, the strategy of this virus is indivisibly linked with its pathogenesis, providing a clue for prevention and treatment of HTLV-1-induced diseases.This article is part of the themed issue 'Human oncogenic viruses'.
Keywords: HBZ; HTLV-1; Tax.
© 2017 The Authors.
Conflict of interest statement
We have no competing interests.
Figures

References
-
- Reid MJ, et al. 2016. Detailed phylogenetic analysis of primate T-lymphotropic virus type 1 (PTLV-1) sequences from orangutans (Pongo pygmaeus) reveals new insights into the evolutionary history of PTLV-1 in Asia. Infect. Genet. Evol. 43, 434–450. ( 10.1016/j.meegid.2016.05.036) - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
