Epithelial-mesenchymal transition transcription factors control pluripotent adult stem cell migration in vivo in planarians
- PMID: 28893948
- PMCID: PMC5665486
- DOI: 10.1242/dev.154971
Epithelial-mesenchymal transition transcription factors control pluripotent adult stem cell migration in vivo in planarians
Abstract
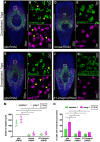
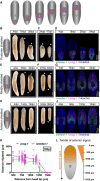
Migration of stem cells underpins the physiology of metazoan animals. For tissues to be maintained, stem cells and their progeny must migrate and differentiate in the correct positions. This need is even more acute after tissue damage by wounding or pathogenic infection. Inappropriate migration also underpins metastasis. Despite this, few mechanistic studies address stem cell migration during repair or homeostasis in adult tissues. Here, we present a shielded X-ray irradiation assay that allows us to follow stem cell migration in planarians. We demonstrate the use of this system to study the molecular control of stem cell migration and show that snail-1, snail-2 and zeb-1 EMT transcription factor homologs are necessary for cell migration to wound sites and for the establishment of migratory cell morphology. We also observed that stem cells undergo homeostatic migration to anterior regions that lack local stem cells, in the absence of injury, maintaining tissue homeostasis. This requires the polarity determinant notum Our work establishes planarians as a suitable model for further in-depth study of the processes controlling stem cell migration in vivo.
Keywords: EMT; Migration; Planarian; Pluripotency; Schmidtea mediterranea; Snail; Wounding.
© 2017. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures





References
-
- Adhikary A., Chakraborty S., Mazumdar M., Ghosh S., Mukherjee S., Manna A., Mohanty S., Nakka K. K., Joshi S., De A. et al. (2014). Inhibition of epithelial to mesenchymal transition by E-cadherin up-regulation via repression of slug transcription and inhibition of E-cadherin degradation: dual role of scaffold/matrix attachment region-binding protein 1 (SMAR1) in breast cancer cells. J. Biol. Chem. 289, 25431-25444. 10.1074/jbc.M113.527267 - DOI - PMC - PubMed
-
- Bardeen C. R. and Baetjer F. H. (1904). The inhibitive action of the Roentgen rays on regeneration in planarians. J. Exp. Zool. 1, 191-195. 10.1002/jez.1400010107 - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

