Agonist-Dependent and -Independent κ Opioid Receptor Phosphorylation: Distinct Phosphorylation Patterns and Different Cellular Outcomes
- PMID: 28893975
- PMCID: PMC5635518
- DOI: 10.1124/mol.117.108555
Agonist-Dependent and -Independent κ Opioid Receptor Phosphorylation: Distinct Phosphorylation Patterns and Different Cellular Outcomes
Erratum in
-
Correction to "Agonist-Dependent and-Independent κ Opioid Receptor Phosphorylation: Distinct Phosphorylation Patterns and Different Cellular Outcomes".Mol Pharmacol. 2018 Aug;94(2):895. doi: 10.1124/mol.117.108555err. Mol Pharmacol. 2018. PMID: 29945897 Free PMC article. No abstract available.
Abstract
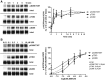
We reported previously that the selective agonist U50,488H promoted phosphorylation of the mouse κ opioid receptor (KOPR) at residues S356, T357, T363, and S369. Here, we found that agonist (U50,488H)-dependent KOPR phosphorylation at all the residues was mediated by Gi/o α proteins and multiple protein kinases [GRK2, GRK3, GRK5, GRK6 and protein kinase C (PKC)]. In addition, PKC activation by phorbol ester induced agonist-independent KOPR phosphorylation. Compared with U50,488H, PKC activation promoted much higher S356/T357 phosphorylation, much lower T363 phosphorylation, and similar levels of S369 phosphorylation. After U50,488H treatment, GRKs, but not PKC, were involved in agonist-induced KOPR internalization. In contrast, PKC activation caused a lower level of agonist-independent KOPR internalization, compared with U50,488H. U50,488H-induced activation of extracellular signal-regulated kinase 1/2 (ERK1/2) was G protein-, but not β-arrestin-, dependent. After U50,488H treatment, GRK-mediated, but not PKC-mediated, KOPR phosphorylation followed by β-arrestin recruitment desensitized U50,488H-induced ERK1/2 response. Therefore, agonist-dependent (GRK- and PKC-mediated) and agonist-independent (PKC-promoted) KOPR phosphorylations show distinct phosphorylation patterns, leading to diverse cellular outcomes.
Copyright © 2017 by The American Society for Pharmacology and Experimental Therapeutics.
Figures








References
-
- Ansonoff MA, Zhang J, Czyzyk T, Rothman RB, Stewart J, Xu H, Zjwiony J, Siebert DJ, Yang F, Roth BL, et al. (2006) Antinociceptive and hypothermic effects of Salvinorin A are abolished in a novel strain of kappa-opioid receptor-1 knockout mice. J Pharmacol Exp Ther 318:641–648. - PubMed
-
- Appleyard SM, Celver J, Pineda V, Kovoor A, Wayman GA, Chavkin C. (1999) Agonist-dependent desensitization of the kappa opioid receptor by G protein receptor kinase and beta-arrestin. J Biol Chem 274:23802–23807. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

