Cytokinin-Auxin Crosstalk in the Gynoecial Primordium Ensures Correct Domain Patterning
- PMID: 28894023
- PMCID: PMC5664465
- DOI: 10.1104/pp.17.00805
Cytokinin-Auxin Crosstalk in the Gynoecial Primordium Ensures Correct Domain Patterning
Abstract
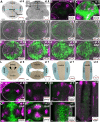
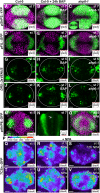
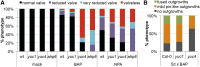
The Arabidopsis (Arabidopsis thaliana) gynoecium consists of two congenitally fused carpels made up of two lateral valve domains and two medial domains, which retain meristematic properties and later fuse to produce the female reproductive structures vital for fertilization. Polar auxin transport (PAT) is important for setting up distinct apical auxin signaling domains in the early floral meristem remnants allowing for lateral domain identity and outgrowth. Crosstalk between auxin and cytokinin plays an important role in the development of other meristematic tissues, but hormone interaction studies to date have focused on more accessible later-stage gynoecia and the spatiotemporal interactions pivotal for patterning of early gynoecium primordia remain unknown. Focusing on the earliest stages, we propose a cytokinin-auxin feedback model during early gynoecium patterning and hormone homeostasis. Our results suggest that cytokinin positively regulates auxin signaling in the incipient gynoecial primordium and strengthen the concept that cytokinin regulates auxin homeostasis during gynoecium development. Specifically, medial cytokinin promotes auxin biosynthesis components [YUCCA1/4 (YUC1/4)] in, and PINFORMED7 (PIN7)-mediated auxin efflux from, the medial domain. The resulting laterally focused auxin signaling triggers ARABIDOPSIS HISTIDINE PHOSPHOTRANSFER PROTEIN6 (AHP6), which then represses cytokinin signaling in a PAT-dependent feedback. Cytokinin also down-regulates PIN3, promoting auxin accumulation in the apex. The yuc1, yuc4, and ahp6 mutants are hypersensitive to exogenous cytokinin and 1-napthylphthalamic acid (NPA), highlighting their role in mediolateral gynoecium patterning. In summary, these mechanisms self-regulate cytokinin and auxin signaling domains, ensuring correct domain specification and gynoecium development.
© 2017 American Society of Plant Biologists. All Rights Reserved.
Figures



Similar articles
-
The bHLH transcription factor SPATULA enables cytokinin signaling, and both activate auxin biosynthesis and transport genes at the medial domain of the gynoecium.PLoS Genet. 2017 Apr 7;13(4):e1006726. doi: 10.1371/journal.pgen.1006726. eCollection 2017 Apr. PLoS Genet. 2017. PMID: 28388635 Free PMC article.
-
Polar auxin transport is essential for medial versus lateral tissue specification and vascular-mediated valve outgrowth in Arabidopsis gynoecia.Plant Physiol. 2014 Dec;166(4):1998-2012. doi: 10.1104/pp.114.245951. Epub 2014 Oct 20. Plant Physiol. 2014. PMID: 25332506 Free PMC article.
-
Auxin and cytokinin act during gynoecial patterning and the development of ovules from the meristematic medial domain.Wiley Interdiscip Rev Dev Biol. 2015 Nov-Dec;4(6):555-71. doi: 10.1002/wdev.193. Epub 2015 May 7. Wiley Interdiscip Rev Dev Biol. 2015. PMID: 25951007 Review.
-
TCP15 modulates cytokinin and auxin responses during gynoecium development in Arabidopsis.Plant J. 2015 Oct;84(2):267-82. doi: 10.1111/tpj.12992. Plant J. 2015. PMID: 26303297
-
Auxin and the Arabidopsis thaliana gynoecium.J Exp Bot. 2013 Jun;64(9):2619-27. doi: 10.1093/jxb/ert099. Epub 2013 Apr 12. J Exp Bot. 2013. PMID: 23585670 Review.
Cited by
-
Auxin regulation involved in gynoecium morphogenesis of papaya flowers.Hortic Res. 2019 Nov 1;6:119. doi: 10.1038/s41438-019-0205-8. eCollection 2019. Hortic Res. 2019. PMID: 31700646 Free PMC article.
-
Auxin and cytokinin coordinate the dormancy and outgrowth of axillary bud in strawberry runner.BMC Plant Biol. 2019 Nov 29;19(1):528. doi: 10.1186/s12870-019-2151-x. BMC Plant Biol. 2019. PMID: 31783789 Free PMC article.
-
Antagonistic activity of auxin and cytokinin in shoot and root organs.Plant Direct. 2019 Feb 25;3(2):e00121. doi: 10.1002/pld3.121. eCollection 2019 Feb. Plant Direct. 2019. PMID: 31245764 Free PMC article.
-
Coordination of biradial-to-radial symmetry and tissue polarity by HD-ZIP II proteins.Nat Commun. 2021 Jul 14;12(1):4321. doi: 10.1038/s41467-021-24550-6. Nat Commun. 2021. PMID: 34262040 Free PMC article.
-
Hormonal Regulation and Crosstalk of Auxin/Cytokinin Signaling Pathways in Potatoes In Vitro and in Relation to Vegetation or Tuberization Stages.Int J Mol Sci. 2021 Jul 30;22(15):8207. doi: 10.3390/ijms22158207. Int J Mol Sci. 2021. PMID: 34360972 Free PMC article.
References
-
- Benková E, Michniewicz M, Sauer M, Teichmann T, Seifertová D, Jürgens G, Friml J (2003) Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115: 591–602 - PubMed
-
- Besnard F, Refahi Y, Morin V, Marteaux B, Brunoud G, Chambrier P, Rozier F, Mirabet V, Legrand J, Lainé S, Thévenon E, Farcot E, et al. (2014a) Cytokinin signalling inhibitory fields provide robustness to phyllotaxis. Nature 505: 417–421 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

