MTORC1 Regulates both General Autophagy and Mitophagy Induction after Oxidative Phosphorylation Uncoupling
- PMID: 28894028
- PMCID: PMC5686580
- DOI: 10.1128/MCB.00441-17
MTORC1 Regulates both General Autophagy and Mitophagy Induction after Oxidative Phosphorylation Uncoupling
Abstract
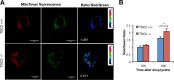
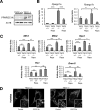
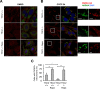
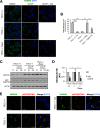
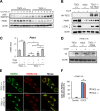
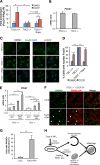
Mechanistic target of rapamycin complex 1 (MTORC1) is a critical negative regulator of general autophagy. We hypothesized that MTORC1 may specifically regulate autophagic clearance of damaged mitochondria. To test this, we used cells lacking tuberous sclerosis complex 2 (TSC2-/- cells), which show constitutive MTORC1 activation. TSC2-/- cells show MTORC1-dependent impaired autophagic flux after chemical uncoupling of mitochondria, increased mitochondrial-protein aging, and accumulation of p62/SQSTM1-positive mitochondria. Mitochondrial autophagy (mitophagy) was also deficient in cells lacking TSC2, associated with altered expression of PTEN-induced putative kinase 1 (PINK1) and PARK2 translocation to uncoupled mitochondria, all of which were recovered by MTORC1 inhibition or expression of constitutively active forkhead box protein O1 (FoxO1). These data prove the necessity of intact MTORC1 signaling to regulate two synergistic processes required for clearance of damaged mitochondria: (i) general autophagy initiation and (ii) PINK1/PARK2-mediated selective targeting of uncoupled mitochondria to the autophagic machinery.
Keywords: MTORC1; PINK1; TSC2; autophagy; mitophagy; rapamycin.
Copyright © 2017 American Society for Microbiology.
Figures

References
-
- Ravikumar B, Sarkar S, Davies JE, Futter M, Garcia-Arencibia M, Green-Thompson ZW, Jimenez-Sanchez M, Korolchuk VI, Lichtenberg M, Luo S, Massey DCO, Menzies FM, Moreau K, Narayanan U, Renna M, Siddiqi FH, Underwood BR, Winslow AR, Rubinsztein DC. 2010. Regulation of mammalian autophagy in physiology and pathophysiology. Physiol Rev 90:1383–1435. doi: 10.1152/physrev.00030.2009. - DOI - PubMed
-
- Ravikumar B, Vacher C, Berger Z, Davies JE, Luo S, Oroz LG, Scaravilli F, Easton DF, Duden R, O'Kane CJ, Rubinsztein DC. 2004. Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat Genet 36:585–595. doi: 10.1038/ng1362. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
