Cinaciguat ameliorates glomerular damage by reducing ERK1/2 activity and TGF-ß expression in type-1 diabetic rats
- PMID: 28894114
- PMCID: PMC5593847
- DOI: 10.1038/s41598-017-10125-3
Cinaciguat ameliorates glomerular damage by reducing ERK1/2 activity and TGF-ß expression in type-1 diabetic rats
Abstract
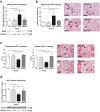
Decreased soluble guanylate cyclase activity and cGMP levels in diabetic kidneys were shown to influence the progression of nephropathy. The regulatory effects of soluble guanylate cyclase activators on renal signaling pathways are still unknown, we therefore investigated the renal molecular effects of the soluble guanylate cyclase activator cinaciguat in type-1 diabetic (T1DM) rats. Male adult Sprague-Dawley rats were divided into 2 groups after induction of T1DM with 60 mg/kg streptozotocin: DM, untreated (DM, n = 8) and 2) DM + cinaciguat (10 mg/kg per os daily, DM-Cin, n = 8). Non-diabetic untreated and cinaciguat treated rats served as controls (Co (n = 10) and Co-Cin (n = 10), respectively). Rats were treated for eight weeks, when renal functional and molecular analyses were performed. Cinaciguat attenuated the diabetes induced proteinuria, glomerulosclerosis and renal collagen-IV expression accompanied by 50% reduction of TIMP-1 expression. Cinaciguat treatment restored the glomerular cGMP content and soluble guanylate cyclase expression, and ameliorated the glomerular apoptosis (TUNEL positive cell number) and podocyte injury. These effects were accompanied by significantly reduced TGF-ß overexpression and ERK1/2 phosphorylation in cinaciguat treated diabetic kidneys. We conclude that the soluble guanylate cyclase activator cinaciguat ameliorated diabetes induced glomerular damage, apoptosis, podocyte injury and TIMP-1 overexpression by suppressing TGF-ß and ERK1/2 signaling.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures






Similar articles
-
Activation of soluble guanylyl cyclase signalling with cinaciguat improves impaired kidney function in diabetic mice.Br J Pharmacol. 2022 Jun;179(11):2460-2475. doi: 10.1111/bph.15425. Epub 2021 May 4. Br J Pharmacol. 2022. PMID: 33651375
-
Targeted Delivery of Soluble Guanylate Cyclase (sGC) Activator Cinaciguat to Renal Mesangial Cells via Virus-Mimetic Nanoparticles Potentiates Anti-Fibrotic Effects by cGMP-Mediated Suppression of the TGF-β Pathway.Int J Mol Sci. 2021 Mar 4;22(5):2557. doi: 10.3390/ijms22052557. Int J Mol Sci. 2021. PMID: 33806499 Free PMC article.
-
Resveratrol ameliorates early diabetic nephropathy associated with suppression of augmented TGF-β/smad and ERK1/2 signaling in streptozotocin-induced diabetic rats.Chem Biol Interact. 2011 Mar 15;190(1):45-53. doi: 10.1016/j.cbi.2011.01.033. Epub 2011 Feb 22. Chem Biol Interact. 2011. PMID: 21300041
-
Selective phosphodiesterase-5 (PDE-5) inhibitor vardenafil ameliorates renal damage in type 1 diabetic rats by restoring cyclic 3',5' guanosine monophosphate (cGMP) level in podocytes.Nephrol Dial Transplant. 2013 Jul;28(7):1751-61. doi: 10.1093/ndt/gfs391. Epub 2012 Nov 29. Nephrol Dial Transplant. 2013. PMID: 23203993
-
Cinaciguat in combination with insulin induces a favorable effect on implant osseointegration in type 2 diabetic rats.Biomed Pharmacother. 2019 Oct;118:109216. doi: 10.1016/j.biopha.2019.109216. Epub 2019 Jul 15. Biomed Pharmacother. 2019. PMID: 31319371
Cited by
-
Exploring molecular targets in diabetic kidney disease.Kidney Res Clin Pract. 2022 Sep;41(Suppl 2):S33-S45. doi: 10.23876/j.krcp.21.251. Epub 2022 Mar 15. Kidney Res Clin Pract. 2022. PMID: 35354246 Free PMC article.
-
Effects of soluble guanylate cyclase stimulator on renal function in ZSF-1 model of diabetic nephropathy.PLoS One. 2022 Jan 27;17(1):e0261000. doi: 10.1371/journal.pone.0261000. eCollection 2022. PLoS One. 2022. PMID: 35085251 Free PMC article.
-
Oxidative Stress: A Culprit in the Progression of Diabetic Kidney Disease.Antioxidants (Basel). 2024 Apr 12;13(4):455. doi: 10.3390/antiox13040455. Antioxidants (Basel). 2024. PMID: 38671903 Free PMC article. Review.
-
Comparison of sGC activator and sGC stimulator in 5/6 nephrectomized rats on high-salt-diet.Front Pharmacol. 2024 Oct 18;15:1480186. doi: 10.3389/fphar.2024.1480186. eCollection 2024. Front Pharmacol. 2024. PMID: 39494352 Free PMC article.
-
Repurposing Riociguat to Target a Novel Paracrine Nitric Oxide-TRPC6 Pathway to Prevent Podocyte Injury.Int J Mol Sci. 2021 Nov 19;22(22):12485. doi: 10.3390/ijms222212485. Int J Mol Sci. 2021. PMID: 34830371 Free PMC article.
References
-
- Suzuki D, et al. Immunohistochemical evidence for an increased oxidative stress and carbonyl modification of proteins in diabetic glomerular lesions. Journal of the American Society of Nephrology: JASN. 1999;10:822–832. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

