The PNKD gene is associated with Tourette Disorder or Tic disorder in a multiplex family
- PMID: 28894297
- PMCID: PMC5847395
- DOI: 10.1038/mp.2017.179
The PNKD gene is associated with Tourette Disorder or Tic disorder in a multiplex family
Abstract
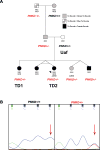
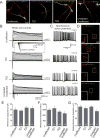
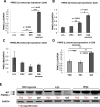
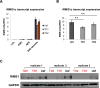
Tourette Disorder (TD) is a childhood-onset neuropsychiatric and neurodevelopmental disorder characterized by the presence of both motor and vocal tics. The genetic architecture of TD is believed to be complex and heterogeneous. Nevertheless, DNA sequence variants co-segregating with TD phenotypes within multiplex families have been identified. This report examines whole exomes of affected and unaffected individuals in a multiplex TD family to discover genes involved in the TD etiology. We performed whole exome sequencing on six out of nine members in a three-generation TD multiplex family. Putative deleterious sequence variants co-segregating with TD patients were identified by our in-house bioinformatics pipeline. Induced pluripotent stem cells (iPSCs) were generated from one unaffected and two TD affected individuals. Neurons were derived from the iPSCs and biochemical assays were conducted to evaluate possible molecular differences between affected and unaffected. A rare heterozygous nonsense mutation in PNKD was co-segregated with TD in this multiplex family. Transcript and protein levels of the PNKD long isoform were reduced in neurons derived from the individuals with TD due to the nonsense mutation, indicating nonsense-mediated mRNA decay. We demonstrated that the PNKD long isoform monomer oligomerizes with itself as well as interacts with the synaptic active zone protein RIMS1α. We concluded that reduced PNKD long isoform levels are detected in all affected individuals and we provide evidence for a mechanism whereby this might contribute to the TD phenotype.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Abelson JF, Kwan KY, O’Roak BJ, Baek DY, Stillman AA, Morgan TM, et al. Sequence variants in SLITRK1 are associated with Tourette’s syndrome. Science. 2005;310(5746):317–320. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

