Gβγ subunit activation promotes dopamine efflux through the dopamine transporter
- PMID: 28894302
- PMCID: PMC5996372
- DOI: 10.1038/mp.2017.176
Gβγ subunit activation promotes dopamine efflux through the dopamine transporter
Abstract
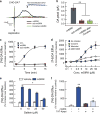
The dopamine transporter (DAT) is an important regulator of brain dopamine (DA) homeostasis, controlling the intensity and duration of DA signaling. DAT is the target for psychostimulants-like cocaine and amphetamine-and plays an important role in neuropsychiatric disorders, including attention-deficit hyperactivity disorder and drug addiction. Thus, a thorough understanding of the mechanisms that regulate DAT function is necessary for the development of clinical interventions to treat DA-related brain disorders. Previous studies have revealed a plethora of protein-protein interactions influencing DAT cellular localization and activity, suggesting that the fine-tuning of DA homeostasis involves multiple mechanisms. We recently reported that G-protein beta-gamma (Gβγ) subunits bind directly to DAT and decrease DA clearance. Here we show that Gβγ induces the release of DA through DAT. Specifically, a Gβγ-binding/activating peptide, mSIRK, increases DA efflux through DAT in heterologous cells and primary dopaminergic neurons in culture. Addition of the Gβγ inhibitor gallein or DAT inhibitors prevents this effect. Residues 582 to 596 in the DAT carboxy terminus were identified as the primary binding site of Gβγ. A TAT peptide containing the Gβγ-interacting domain of DAT blocked the ability of mSIRK to induce DA efflux, consistent with a direct interaction of Gβγ with the transporter. Finally, activation of a G-protein-coupled receptor, the muscarinic M5R, results in DAT-mediated DA efflux through a Gβγ-dependent mechanism. Collectively, our data show that Gβγ interacts with DAT to promote DA efflux. This novel mechanism may have important implications in the regulation of brain DA homeostasis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


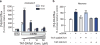
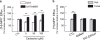
References
-
- Torres GE, Gainetdinov RR, Caron MG. Plasma membrane monoamine transporters: structure, regulation and function. Nat Rev Neurosci. 2003;4:13–25. - PubMed
-
- Cragg SJ, Rice ME. DAncing past the DAT at a DA synapse. Trends Neurosci. 2004;27:270–277. - PubMed
-
- Giros B, Caron MG. Molecular characterization of the dopamine transporter. Trends Pharmacol Sci. 1993;14:43–49. - PubMed
-
- Sulzer D, Sonders MS, Poulsen NW, Galli A. Mechanisms of neurotransmitter release by amphetamines: a review. Prog Neurobiol. 2005;75:406–433. - PubMed
-
- Robbins TW, Everitt BJ. Drug addiction: bad habits add up. Nature. 1999;398:567–570. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

