Challenges in translational drug research in neuropathic and inflammatory pain: the prerequisites for a new paradigm
- PMID: 28894907
- PMCID: PMC5599481
- DOI: 10.1007/s00228-017-2301-8
Challenges in translational drug research in neuropathic and inflammatory pain: the prerequisites for a new paradigm
Abstract
Aim: Despite an improved understanding of the molecular mechanisms of nociception, existing analgesic drugs remain limited in terms of efficacy in chronic conditions, such as neuropathic pain. Here, we explore the underlying pathophysiological mechanisms of neuropathic and inflammatory pain and discuss the prerequisites and opportunities to reduce attrition and high-failure rate in the development of analgesic drugs.
Methods: A literature search was performed on preclinical and clinical publications aimed at the evaluation of analgesic compounds using MESH terms in PubMed. Publications were selected, which focused on (1) disease mechanisms leading to chronic/neuropathic pain and (2) druggable targets which are currently under evaluation in drug development. Attention was also given to the role of biomarkers and pharmacokinetic-pharmacodynamic modelling.
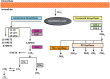
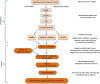
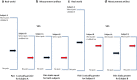
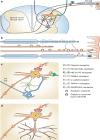
Results: Multiple mechanisms act concurrently to produce pain, which is a non-specific manifestation of underlying nociceptive pathways. Whereas these manifestations can be divided into neuropathic and inflammatory pain, it is now clear that inflammatory mechanisms are a common trigger for both types of pain. This has implications for drug development, as the assessment of drug effects in experimental models of neuropathic and chronic pain is driven by overt behavioural measures. By contrast, the use of mechanistic biomarkers in inflammatory pain has provided the pharmacological basis for dose selection and evaluation of non-steroidal anti-inflammatory drugs (NSAIDs).
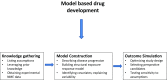
Conclusion: A different paradigm is required for the identification of relevant targets and candidate molecules whereby pain is coupled to the cause of sensorial signal processing dysfunction rather than clinical symptoms. Biomarkers which enable the characterisation of drug binding and target activity are needed for a more robust dose rationale in early clinical development. Such an approach may be facilitated by quantitative clinical pharmacology and evolving technologies in brain imaging, allowing accurate assessment of target engagement, and prediction of treatment effects before embarking on large clinical trials.
Keywords: Analgesics; Chronic pain; Drug development; Hyperalgesia; Inflammatory pain; Neuropathic pain; PKPD modelling.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

