Delayed Activation Kinetics of Th2- and Th17 Cells Compared to Th1 Cells
- PMID: 28895901
- PMCID: PMC5617975
- DOI: 10.3390/cells6030029
Delayed Activation Kinetics of Th2- and Th17 Cells Compared to Th1 Cells
Abstract
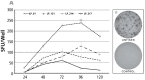
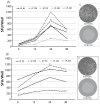
During immune responses, different classes of T cells arise: Th1, Th2, and Th17. Mobilizing the right class plays a critical role in successful host defense and therefore defining the ratios of Th1/Th2/Th17 cells within the antigen-specific T cell repertoire is critical for immune monitoring purposes. Antigen-specific Th1, Th2, and Th17 cells can be detected by challenging peripheral blood mononuclear cells (PBMC) with antigen, and establishing the numbers of T cells producing the respective lead cytokine, IFN-γ and IL-2 for Th1 cells, IL-4 and IL-5 for Th2, and IL-17 for Th-17 cells, respectively. Traditionally, these cytokines are measured within 6 h in flow cytometry. We show here that 6 h of stimulation is sufficient to detect peptide-induced production of IFN-γ, but 24 h are required to reveal the full frequency of protein antigen-specific Th1 cells. Also the detection of IL-2 producing Th1 cells requires 24 h stimulation cultures. Measurements of IL-4 producing Th2 cells requires 48-h cultures and 96 h are required for frequency measurements of IL-5 and IL-17 secreting T cells. Therefore, accounting for the differential secretion kinetics of these cytokines is critical for the accurate determination of the frequencies and ratios of antigen-specific Th1, Th2, and Th17 cells.
Keywords: CD4 cells; ELISPOT; T cells; cytokine kinetics; immune monitoring; multiplexing.
Conflict of interest statement
P.V.L. is Founder, President and CEO of CTL, a company that specializes in immune monitoring by ELISPOT. A.D and A.P. are visiting scholars at CTL. The authors declare no conflicts of interest pertaining to this study.
Figures






References
-
- Macatonia S.E., Hosken N.A., Litton M., Vieira P., Hsieh C.S., Culpepper J.A., Wysocka M., Trinchieri G., Murphy K.M., O’Garra A. Dendritic cells produce il-12 and direct the development of th1 cells from naive cd4+ t cells. J. Immunol. 1995;154:5071–5079. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

