Rapid and Highly Sensitive Non-Competitive Immunoassay for Specific Detection of Nodularin
- PMID: 28895936
- PMCID: PMC5620649
- DOI: 10.3390/microorganisms5030058
Rapid and Highly Sensitive Non-Competitive Immunoassay for Specific Detection of Nodularin
Abstract
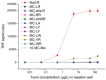
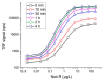
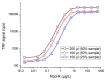
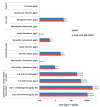
Nodularin (NOD) is a cyclic penta-peptide hepatotoxin mainly produced by Nodularia spumigena, reported from the brackish water bodies of various parts of the world. It can accumulate in the food chain and, for safety reasons, levels of NOD not only in water bodies but also in food matrices are of interest. Here, we report on a non-competitive immunoassay for the specific detection of NOD. A phage display technique was utilized to interrogate a synthetic antibody phage library for binders recognizing NOD bound to an anti-ADDA (3-Amino-9-methoxy-2,6,8-trimethyl-10-phenyldeca-4(E),6(E)-dienoic acid) monoclonal antibody (Mab). One of the obtained immunocomplex binders, designated SA32C11, showed very high specificity towards nodularin-R (NOD-R) over to the tested 10 different microcystins (microcystin-LR, -dmLR, -RR, -dmRR, -YR, -LY, -LF, -LW, -LA, -WR). It was expressed in Escherichia coli as a single chain antibody fragment (scFv) fusion protein and used to establish a time-resolved fluorometry-based assay in combination with the anti-ADDA Mab. The detection limit (blank + 3SD) of the immunoassay, with a total assay time of 1 h 10 min, is 0.03 µg/L of NOD-R. This represents the most sensitive immunoassay method for the specific detection of NOD reported so far. The assay was tested for its performance to detect NOD using spiked (0.1 to 3 µg/L of NOD-R) water samples including brackish sea and coastal water and the recovery ranged from 79 to 127%. Furthermore, a panel of environmental samples, including water from different sources, fish and other marine tissue specimens, were analyzed for NOD using the assay. The assay has potential as a rapid screening tool for the analysis of a large number of water samples for the presence of NOD. It can also find applications in the analysis of the bioaccumulation of NOD in marine organisms and in the food chain.
Keywords: anti-immunocomplex binder; cyanotoxin; hepatotoxin; nodularin; non-competitive immunoassay; quantification of nodularin; synthetic antibody phage library.
Conflict of interest statement
S.A., M.V. and U.L. are inventors in a pending patent application PCT/FI2016/050911 concerning the anti-immunocomplex antibody described in the manuscript. The assignee of the application is University of Turku.
Figures
References
-
- Sipiä V., Kankaanpää H., Peltonen H., Vinni M., Meriluoto J. Transfer of nodularin to three-spined stickleback (Gasterosteus aculeatus L.), herring (Clupea harengus L.), and salmon (Salmo salar L.) in the northern baltic sea. Ecotoxicol. Environ. Saf. 2007;66:421–425. doi: 10.1016/j.ecoenv.2006.02.006. - DOI - PubMed
-
- Sipiä V.O., Kankaanpää H.T., Pflugmacher S., Flinkman J., Furey A., James K.J. Bioaccumulation and detoxication of nodularin in tissues of flounder (Platichthys flesus), mussels (Mytilus edulis, Dreissena polymorpha), and clams (Macoma balthica) from the northern baltic sea. Ecotoxicol. Environ. Saf. 2002;53:305–311. doi: 10.1006/eesa.2002.2222. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

