Impact of common genetic determinants of Hemoglobin A1c on type 2 diabetes risk and diagnosis in ancestrally diverse populations: A transethnic genome-wide meta-analysis
- PMID: 28898252
- PMCID: PMC5595282
- DOI: 10.1371/journal.pmed.1002383
Impact of common genetic determinants of Hemoglobin A1c on type 2 diabetes risk and diagnosis in ancestrally diverse populations: A transethnic genome-wide meta-analysis
Abstract
Background: Glycated hemoglobin (HbA1c) is used to diagnose type 2 diabetes (T2D) and assess glycemic control in patients with diabetes. Previous genome-wide association studies (GWAS) have identified 18 HbA1c-associated genetic variants. These variants proved to be classifiable by their likely biological action as erythrocytic (also associated with erythrocyte traits) or glycemic (associated with other glucose-related traits). In this study, we tested the hypotheses that, in a very large scale GWAS, we would identify more genetic variants associated with HbA1c and that HbA1c variants implicated in erythrocytic biology would affect the diagnostic accuracy of HbA1c. We therefore expanded the number of HbA1c-associated loci and tested the effect of genetic risk-scores comprised of erythrocytic or glycemic variants on incident diabetes prediction and on prevalent diabetes screening performance. Throughout this multiancestry study, we kept a focus on interancestry differences in HbA1c genetics performance that might influence race-ancestry differences in health outcomes.
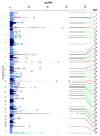
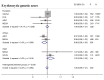
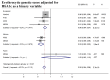
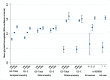
Methods & findings: Using genome-wide association meta-analyses in up to 159,940 individuals from 82 cohorts of European, African, East Asian, and South Asian ancestry, we identified 60 common genetic variants associated with HbA1c. We classified variants as implicated in glycemic, erythrocytic, or unclassified biology and tested whether additive genetic scores of erythrocytic variants (GS-E) or glycemic variants (GS-G) were associated with higher T2D incidence in multiethnic longitudinal cohorts (N = 33,241). Nineteen glycemic and 22 erythrocytic variants were associated with HbA1c at genome-wide significance. GS-G was associated with higher T2D risk (incidence OR = 1.05, 95% CI 1.04-1.06, per HbA1c-raising allele, p = 3 × 10-29); whereas GS-E was not (OR = 1.00, 95% CI 0.99-1.01, p = 0.60). In Europeans and Asians, erythrocytic variants in aggregate had only modest effects on the diagnostic accuracy of HbA1c. Yet, in African Americans, the X-linked G6PD G202A variant (T-allele frequency 11%) was associated with an absolute decrease in HbA1c of 0.81%-units (95% CI 0.66-0.96) per allele in hemizygous men, and 0.68%-units (95% CI 0.38-0.97) in homozygous women. The G6PD variant may cause approximately 2% (N = 0.65 million, 95% CI 0.55-0.74) of African American adults with T2D to remain undiagnosed when screened with HbA1c. Limitations include the smaller sample sizes for non-European ancestries and the inability to classify approximately one-third of the variants. Further studies in large multiethnic cohorts with HbA1c, glycemic, and erythrocytic traits are required to better determine the biological action of the unclassified variants.
Conclusions: As G6PD deficiency can be clinically silent until illness strikes, we recommend investigation of the possible benefits of screening for the G6PD genotype along with using HbA1c to diagnose T2D in populations of African ancestry or groups where G6PD deficiency is common. Screening with direct glucose measurements, or genetically-informed HbA1c diagnostic thresholds in people with G6PD deficiency, may be required to avoid missed or delayed diagnoses.
Conflict of interest statement
We have read the journal's policy and the authors of this manuscript have the following competing interests: AYC is an employee of Merck, however all work for the manuscript was completed before the start of employment. CEE is a current employee of AstraZeneca. CLan receives a stipend as a specialty consulting editor for PLOS Medicine and serves on the journal's editorial board. EI is a scientific advisor for Precision Wellness, Cellink and Olink Proteomics for work unrelated to the present project. GKH declared institution support from Amgen, AstraZeneca, Cerenis, Ionis, Regeneron Pharmaceuticals, Inc. and Sanofi, Synageva. He has served as a consultant and received speaker fees from Aegerion, Amgen, Sanofi, Regeneron Pharmaceuticals, Inc., and Pfizer. IB and spouse own stock in GlaxoSmithKline and Incyte Corporation. JD declared grants from the National Heart, Lung, and Blood Institute (NHLBI) of the National Institute of Health (NIH) during the course of this study. JIR declared funding from NIH grants. MAN consults for Illumina Inc, the Michael J. Fox Foundation and University of California Healthcare among others. MBl receives speaker’s honoraria and/or compensation for participation in advisory boards from: Astra Zeneca, Bayer, Boehringer-Ingelheim, Lilly, Novo Nordisk, Novartis, MSD, Pfizer, Riemser and Sanofi. MIM was a member of the editorial board of PLOS Medicine at the time this manuscript was submitted. RAS is an employee and shareholder in GlaxoSmithKline.
Figures

References
-
- Franklin CS, Aulchenko YS, Huffman JE, Vitart V, Hayward C, Polasek O, et al. The TCF7L2 diabetes risk variant is associated with HbA(1)(C) levels: a genome-wide association meta-analysis. Ann Hum Genet. 2010;74(6):471–8. doi: 10.1111/j.1469-1809.2010.00607.x . - DOI - PubMed
-
- Pare G, Chasman DI, Parker AN, Nathan DM, Miletich JP, Zee RY, et al. Novel association of HK1 with glycated hemoglobin in a non-diabetic population: a genome-wide evaluation of 14,618 participants in the Women's Genome Health Study. PLoS Genet. 2008;4(12):e1000312 doi: 10.1371/journal.pgen.1000312 ; PubMed Central PMCID: PMC2596965. - DOI - PMC - PubMed
-
- Soranzo N, Sanna S, Wheeler E, Gieger C, Radke D, Dupuis J, et al. Common variants at 10 genomic loci influence hemoglobin A(1)(C) levels via glycemic and nonglycemic pathways. Diabetes. 2010;59(12):3229–39. doi: 10.2337/db10-0502 ; PubMed Central PMCID: PMC2992787. - DOI - PMC - PubMed
-
- Chen P, Takeuchi F, Lee JY, Li H, Wu JY, Liang J, et al. Multiple Non-glycemic Genomic Loci Are Newly Associated with Blood Level of Glycated Hemoglobin in East Asians. Diabetes. 2014. doi: 10.2337/db13-1815 . - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01 HG007417/HG/NHGRI NIH HHS/United States
- MR/K002414/1/MRC_/Medical Research Council/United Kingdom
- R01 DK106236/DK/NIDDK NIH HHS/United States
- R01 DK089174/DK/NIDDK NIH HHS/United States
- MC_UU_12019/1/MRC_/Medical Research Council/United Kingdom
- P30 DK079626/DK/NIDDK NIH HHS/United States
- UL1 TR000124/TR/NCATS NIH HHS/United States
- S10 OD018522/OD/NIH HHS/United States
- K24 DK106414/DK/NIDDK NIH HHS/United States
- K24 DK080140/DK/NIDDK NIH HHS/United States
- U01 DK062370/DK/NIDDK NIH HHS/United States
- MR/K013351/1/MRC_/Medical Research Council/United Kingdom
- U54 GM115428/GM/NIGMS NIH HHS/United States
- RG/10/12/28456/BHF_/British Heart Foundation/United Kingdom
- MC_UU_12015/1/MRC_/Medical Research Council/United Kingdom
- R01 HL105756/HL/NHLBI NIH HHS/United States
- P30 DK063491/DK/NIDDK NIH HHS/United States
- MC_UU_12015/2/MRC_/Medical Research Council/United Kingdom
- P30 DK020541/DK/NIDDK NIH HHS/United States
- MC_PC_U127561128/MRC_/Medical Research Council/United Kingdom
- UM1 CA182913/CA/NCI NIH HHS/United States
- U01 DK078616/DK/NIDDK NIH HHS/United States
- U24 AG051129/AG/NIA NIH HHS/United States
- P30 DK020572/DK/NIDDK NIH HHS/United States
- RG/14/5/30893/BHF_/British Heart Foundation/United Kingdom
- UL1 TR001881/TR/NCATS NIH HHS/United States
- G0601261/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

