Structural investigation of C6/36 and Vero cell cultures infected with a Brazilian Zika virus
- PMID: 28898286
- PMCID: PMC5595330
- DOI: 10.1371/journal.pone.0184397
Structural investigation of C6/36 and Vero cell cultures infected with a Brazilian Zika virus
Abstract
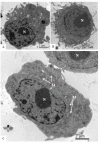
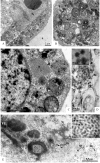
Zika virus (ZIKV) is a member of the flavivirus genus, and its genome is approximately 10.8 kilobases of positive-strand RNA enclosed in a capsid and surrounded by a membrane. Studies on the replication dynamics of ZIKV are scarce, which limits the development of antiviral agents and vaccines directed against ZIKV. In this study, Aedes albopictus mosquito lineage cells (C6/36 cells) and African green monkey kidney epithelial cells (Vero cells) were inoculated with a ZIKV sample isolated from a Brazilian patient, and the infection was characterized by immunofluorescence staining, phase contrast light microscopy, transmission electron microscopy and real-time RT-PCR. The infection was observed in both cell lineages, and ZIKV particles were observed inside lysosomes, the rough endoplasmic reticulum and viroplasm-like structures. The susceptibility of C6/36 and Vero cells to ZIKV infection was demonstrated. Moreover, this study showed that part of the replicative cycle may occur within viroplasm-like structures, which has not been previously demonstrated in other flaviviruses.
Conflict of interest statement
Figures


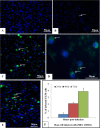

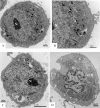
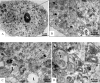
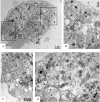

References
-
- Dick GWA, Kitchen SF, Haddow AJ. Zika virus. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg. 1952; 46:509–520. - PubMed
-
- Altman LK (3 July 2007)."Little-Known Virus Challenges a Far-Flung Health System"The New York Times.
-
- Lazear HM, Diamond MS. Zika Virus: New Clinical Syndromes and Its Emergence in the Western Hemisphere. J Virol. 2016; 90(10):4864–75. doi: 10.1128/JVI.00252-16 - DOI - PMC - PubMed
-
- WHO. The History of Zika Virus. Aug 1, 2016. http://www.who.int/emergencies/zika-virus/history/en/ (accessed August 1, 2016).
-
- Heukelbach J, Alencar CH, Kelvin AA, de Oliveira WK, Pamplona de Góes Cavalcanti L. Zika virus outbreak in Brazil. J Infect Dev Ctries. 2016; 10(2):116–20. doi: 10.3855/jidc.8217 - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

