Impact of combined sodium chloride and saturated long-chain fatty acid challenge on the differentiation of T helper cells in neuroinflammation
- PMID: 28899400
- PMCID: PMC5596846
- DOI: 10.1186/s12974-017-0954-y
Impact of combined sodium chloride and saturated long-chain fatty acid challenge on the differentiation of T helper cells in neuroinflammation
Abstract
Background: There has been a marked increase in the incidence of autoimmune diseases like multiple sclerosis (MS) in the last decades which is most likely driven by a change in environmental factors. Here, growing evidence suggests that ingredients of a Western diet like high intake of sodium chloride (NaCl) or saturated fatty acids may impact systemic immune responses, thus increasing disease susceptibility. Recently, we have shown that high dietary salt or long-chain fatty acid (LCFA) intake indeed aggravates T helper (Th) cell responses and neuroinflammation.
Methods: Naïve CD4+ T cells were treated with an excess of 40 mM NaCl and/or 250 μM lauric acid (LA) in vitro to analyze effects on Th cell differentiation, cytokine secretion, and gene expression. We employed ex vivo analyses of the model disease murine experimental autoimmune encephalomyelitis (EAE) to investigate whether salt and LCFA may affect disease severity and T cell activation in vivo.
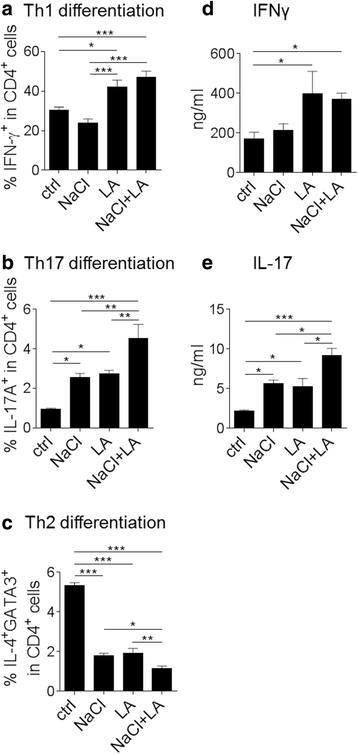
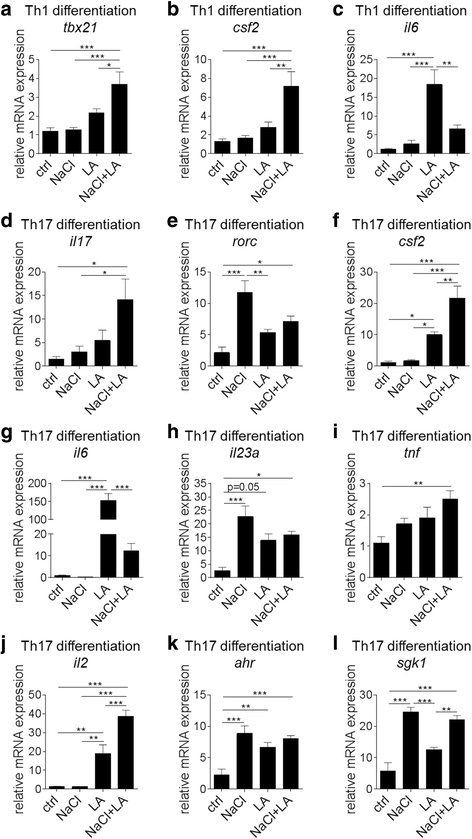
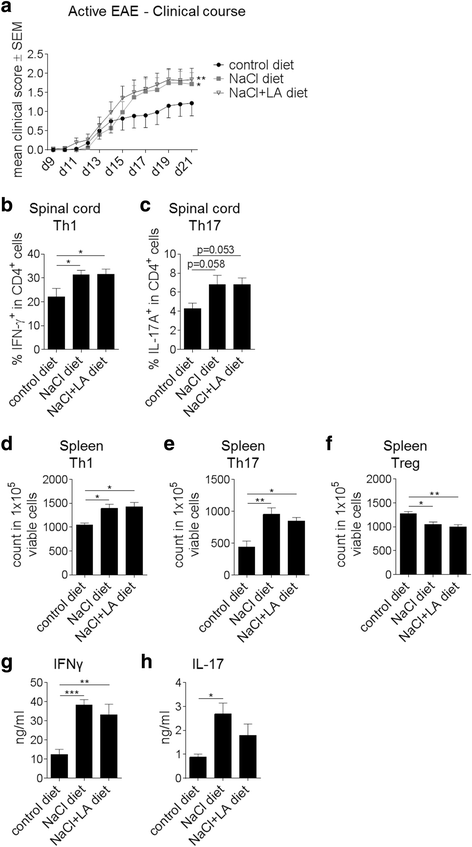
Results: LCFA, like LA, together with NaCl enhance the differentiation of Th1 and Th17 cells as well as pro-inflammatory cytokine and gene expression in vitro. In cell culture, we observed an additive effect of LA and hypertonic extracellular NaCl (NaCl + LA) in Th17 differentiation assays as well as on IL-17, GM-CSF, and IL-2 gene expression. In contrast, NaCl + LA reduced Th2 frequencies. We employed EAE as a model of Th1/Th17 cell-mediated autoimmunity and show that the combination of a NaCl- and LA-rich diet aggravated the disease course and increased T cell infiltration into the central nervous system (CNS) to the same extent as dietary NaCl.
Conclusions: Our findings demonstrate a partially additive effect of NaCl and LA on Th cell polarization in vitro and on Th cell responses in autoimmune neuroinflammation. These data may help to better understand the pathophysiology of autoimmune diseases such as MS.
Keywords: Diet; EAE; Inflammation; Multiple sclerosis; Salt; Saturated fatty acids; T helper cell.
Conflict of interest statement
Ethics approval
All experiments were in accordance with the German laws for animal protection and were approved by local ethic committees (Erlangen AZ 54-2532.1-56/12 and 55.2 DMS 2532-2-27).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- World Health Organization. Atlas: multiple sclerosis resources in the world 2008. 2008. http://www.who.int/iris/handle/10665/43968 of subordinate document. Accessed 9 May 2017.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

