Moderating effects of sex on the impact of diagnosis and amyloid positivity on verbal memory and hippocampal volume
- PMID: 28899422
- PMCID: PMC5596932
- DOI: 10.1186/s13195-017-0300-8
Moderating effects of sex on the impact of diagnosis and amyloid positivity on verbal memory and hippocampal volume
Abstract
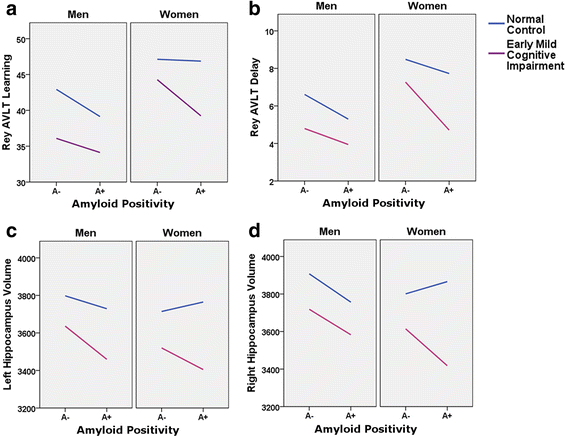
Background: Alzheimer's disease (AD) impacts men and women differently, but the effect of sex on predementia stages is unclear. The objective of this study was to examine whether sex moderates the impact of florbetapir positron emission tomography (PET) amyloid positivity (A+) on verbal learning and memory performance and hippocampal volume (HV) in normal cognition (NC) and early mild cognitive impairment (eMCI).
Methods: Seven hundred forty-two participants with NC and participants with eMCI from the Alzheimer's Disease Neuroimaging Initiative (second cohort [ADNI2] and Grand Opportunity Cohort [ADNI-GO]) were included. All had baseline florbetapir PET measured, and 526 had screening visit HV measured. Regression moderation models were used to examine whether A+ effects on Rey Auditory Verbal Learning Test learning and delayed recall and right and left HV (adjusted for total intracranial volume) were moderated by diagnosis and sex. Age, cognition at screening, education, and apolipoprotein E ε4 carrier status were controlled.
Results: Women with A+, but not those with florbetapir PET amyloid negative (A-),eMCI showed poorer learning. For women with NC, there was no relationship of A+ with learning. In contrast, A+ men trended toward poorer learning regardless of diagnosis. A similar trend was found for verbal delayed recall: Women with A+, but not A-, eMCI trended toward reduced delayed recall; no effects were observed for women with NC or for men. Hippocampal analyses indicated that women with A+, but not those with A-, eMCI, trended toward smaller right HV; no significant A+ effects were observed for women with NC. Men showed similar, though nonsignificant, patterns of smaller right HV in A+ eMCI, but not in men with A- eMCI or NC. No interactive effects of sex were noted for left HV.
Conclusions: Women with NC showed verbal learning and memory scores robust to A+, and women with A+ eMCI lost this advantage. In contrast, A+ impacted men's scores less significantly or not at all, and comparably across those with NC and eMCI. Sex marginally moderated the relationship of A+ and diagnosis with right HV, such that women with NC showed no A+ effect and women with A+ eMCI lost that advantage in neural integrity; the pattern in men was less clear. These findings show that women with A+ eMCI (i.e., prodromal AD) have differential neural and cognitive decline, which has implications for considering sex in early detection of AD and development of therapeutics.
Keywords: Alzheimer’s disease; Amyloid; Memory; Mild cognitive impairment (MCI); PET; Volumetric magnetic resonance imaging (MRI).
Conflict of interest statement
Ethics approval and consent to participate
All data included in this article were collected for research use as part of the Alzheimer’s Disease Neuroimaging Initiative (ADNI) project. ADNI is a longitudinal, multisite AD biomarker study (
Consent for publication
Not applicable.
Competing interests
JZKC, JLB, and SJB declare that they have no competing interests. JLC declares having received in kind research support from Avid Radiopharmaceuticals, Teva Pharmaceuticals, and CogState; having done consultation for AbbVie, ACADIA Pharmaceuticals, Accera, Actinogen Medical, Adamas Pharmaceuticals, Alkahest, Alzheon, Anavex Life Sciences, Astellas Pharma, AstraZeneca, Avanir Pharmaceuticals, Axovant Sciences, Biogen Idec, Biotie Therapies, Boehinger Ingelheim, Chase Pharmaceuticals, Eisai, FORUM Pharmaceuticals, Genentech, Grifols, Intra-Cellular Therapies, Iris Pharma, Ionis Pharmaceuticals, Eli Lilly and Company, Lundbeck, Merck, Neurotrope BioScience, Novartis, Nutricia, Otsuka, Pfizer, Probiodrug, QR Pharma, Resverlogix, Roche, Servier, Sunovion Pharmaceuticals, Suven Life Sciences, Takeda, Taisho Toyama Pharmaceutical Co., Transition Therapeutics, United Neuroscience, GE Healthcare, and MedAvante; owning stock in Adamas Pharmaceuticals, Prana Biotechnology, Sonexa Therapeutics, MedAvante, NeuroTrax, and Neurokos; and owning the copyright of the Neuropsychiatric Inventory. In addition, JLC has provided expert witness/legal consultation regarding olanzapine and ropinerole.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

