NAD+ in Aging: Molecular Mechanisms and Translational Implications
- PMID: 28899755
- PMCID: PMC7494058
- DOI: 10.1016/j.molmed.2017.08.001
NAD+ in Aging: Molecular Mechanisms and Translational Implications
Abstract
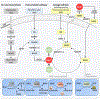
The coenzyme NAD+ is critical in cellular bioenergetics and adaptive stress responses. Its depletion has emerged as a fundamental feature of aging that may predispose to a wide range of chronic diseases. Maintenance of NAD+ levels is important for cells with high energy demands and for proficient neuronal function. NAD+ depletion is detected in major neurodegenerative diseases, such as Alzheimer's and Parkinson's diseases, cardiovascular disease and muscle atrophy. Emerging evidence suggests that NAD+ decrements occur in various tissues during aging, and that physiological and pharmacological interventions bolstering cellular NAD+ levels might retard aspects of aging and forestall some age-related diseases. Here, we discuss aspects of NAD+ biosynthesis, together with putative mechanisms of NAD+ action against aging, including recent preclinical and clinical trials.
Keywords: DNA repair; NAD(+); aging; autophagy; clinical application; metabolism; mitophagy; neurodegenerative disorder; stem cell.
Published by Elsevier Ltd.
Figures
References
-
- Nobel Lectures C−. (1966. (https://www.nobelprize.org/nobel_prizes/chemistry/laureates/1907/buchner...)) Eduard Bunchner-Facts, Elsevier Publishing Company.
-
- Harden A and Young WJ (1906) The alcoholic ferment of yeast-juice. Part II.-The coferment of yeast-juice. Proc. R. Soc. Lond. B (78), 369–375.
-
- James LK (1993) Nobel Laureates in Chemistry 1901–1992, Merck& Co., Inc..
-
- P.D. B(1970) The enzymes (Third edition), Academic Press.
-
- Kornberg A and Pricer WE Jr. (1950) On the structure of triphosphopyridine nucleotide. J Biol Chem 186 (2), 557–67. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

