B cell-derived IL-6 initiates spontaneous germinal center formation during systemic autoimmunity
- PMID: 28899868
- PMCID: PMC5679179
- DOI: 10.1084/jem.20170580
B cell-derived IL-6 initiates spontaneous germinal center formation during systemic autoimmunity
Abstract
Recent studies have identified critical roles for B cells in triggering autoimmune germinal centers (GCs) in systemic lupus erythematosus (SLE) and other disorders. The mechanisms whereby B cells facilitate loss of T cell tolerance, however, remain incompletely defined. Activated B cells produce interleukin 6 (IL-6), a proinflammatory cytokine that promotes T follicular helper (TFH) cell differentiation. Although B cell IL-6 production correlates with disease severity in humoral autoimmunity, whether B cell-derived IL-6 is required to trigger autoimmune GCs has not, to our knowledge, been addressed. Here, we report the unexpected finding that a lack of B cell-derived IL-6 abrogates spontaneous GC formation in mouse SLE, resulting in loss of class-switched autoantibodies and protection from systemic autoimmunity. Mechanistically, B cell IL-6 production was enhanced by IFN-γ, consistent with the critical roles for B cell-intrinsic IFN-γ receptor signals in driving autoimmune GC formation. Together, these findings identify a key mechanism whereby B cells drive autoimmunity via local IL-6 production required for TFH differentiation and autoimmune GC formation.
© 2017 Arkatkar et al.
Figures

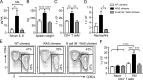
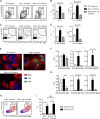

Comment in
-
Systemic lupus erythematosus: B cell-derived IL-6 promotes disease.Nat Rev Rheumatol. 2017 Nov;13(11):633. doi: 10.1038/nrrheum.2017.163. Epub 2017 Sep 28. Nat Rev Rheumatol. 2017. PMID: 28959044 No abstract available.
References
-
- Barr T.A., Shen P., Brown S., Lampropoulou V., Roch T., Lawrie S., Fan B., O’Connor R.A., Anderton S.M., Bar-Or A., et al. . 2012. B cell depletion therapy ameliorates autoimmune disease through ablation of IL-6-producing B cells. J. Exp. Med. 209:1001–1010. 10.1084/jem.20111675 - DOI - PMC - PubMed
-
- Becker-Herman S., Meyer-Bahlburg A., Schwartz M.A., Jackson S.W., Hudkins K.L., Liu C., Sather B.D., Khim S., Liggitt D., Song W., et al. . 2011. WASp-deficient B cells play a critical, cell-intrinsic role in triggering autoimmunity. J. Exp. Med. 208:2033–2042. 10.1084/jem.20110200 - DOI - PMC - PubMed
-
- Bentebibel S.E., Lopez S., Obermoser G., Schmitt N., Mueller C., Harrod C., Flano E., Mejias A., Albrecht R.A., Blankenship D., et al. . 2013. Induction of ICOS+CXCR3+CXCR5+ TH cells correlates with antibody responses to influenza vaccination. Sci. Transl. Med. 5:176ra32 10.1126/scitranslmed.3005191 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

