Pollen Aperture Factor INP1 Acts Late in Aperture Formation by Excluding Specific Membrane Domains from Exine Deposition
- PMID: 28899962
- PMCID: PMC5761771
- DOI: 10.1104/pp.17.00720
Pollen Aperture Factor INP1 Acts Late in Aperture Formation by Excluding Specific Membrane Domains from Exine Deposition
Abstract
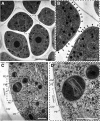
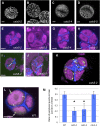
Accurate placement of extracellular materials is a critical part of cellular development. To study how cells achieve this accuracy, we use formation of pollen apertures as a model. In Arabidopsis (Arabidopsis thaliana), three regions on the pollen surface lack deposition of pollen wall exine and develop into apertures. In developing pollen, Arabidopsis INAPERTURATE POLLEN1 (INP1) protein acts as a marker for the preaperture domains, assembling there into three punctate lines. To understand the mechanism of aperture formation, we studied the dynamics of INP1 expression and localization and its relationship with the membrane domains at which it assembles. We found that INP1 assembly occurs after meiotic cytokinesis at the interface between the plasma membrane and the overlying callose wall, and requires the normal callose wall formation. Sites of INP1 localization coincide with positions of protruding membrane ridges in proximity to the callose wall. Our data suggest that INP1 is a late-acting factor involved in keeping specific membrane domains next to the callose wall to prevent formation of exine at these sites.
© 2018 American Society of Plant Biologists. All Rights Reserved.
Figures


References
-
- Alassimone J, Fujita S, Doblas VG, van Dop M, Barberon M, Kalmbach L, Vermeer JEM, Rojas-Murcia N, Santuari L, Hardtke CS, et al. (2016) Polarly localized kinase SGN1 is required for Casparian strip integrity and positioning. Nat Plants 2: 16113. - PubMed
-
- Albert B, Ressayre A, Nadot S (2011) Correlation between pollen aperture pattern and callose deposition in late tetrad stage in three species producing atypical pollen grains. Am J Bot 98: 189–196 - PubMed
-
- Ariizumi T, Toriyama K (2011) Genetic regulation of sporopollenin synthesis and pollen exine development. Annu Rev Plant Biol 62: 437–460 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

