On the origin of shape fluctuations of the cell nucleus
- PMID: 28900009
- PMCID: PMC5625896
- DOI: 10.1073/pnas.1702226114
On the origin of shape fluctuations of the cell nucleus
Abstract
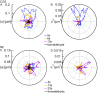
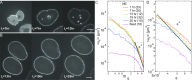
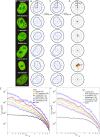
The nuclear envelope (NE) presents a physical boundary between the cytoplasm and the nucleoplasm, sandwiched in between two highly active systems inside the cell: cytoskeleton and chromatin. NE defines the shape and size of the cell nucleus, which increases during the cell cycle, accommodating for chromosome decondensation followed by genome duplication. In this work, we study nuclear shape fluctuations at short time scales of seconds in human cells. Using spinning disk confocal microscopy, we observe fast fluctuations of the NE, visualized by fluorescently labeled lamin A, and of the chromatin globule surface (CGS) underneath the NE, visualized by fluorescently labeled histone H2B. Our findings reveal that fluctuation amplitudes of both CGS and NE monotonously decrease during the cell cycle, serving as a reliable cell cycle stage indicator. Remarkably, we find that, while CGS and NE typically fluctuate in phase, they do exhibit localized regions of out-of-phase motion, which lead to separation of NE and CGS. To explore the mechanism behind these shape fluctuations, we use biochemical perturbations. We find the shape fluctuations of CGS and NE to be both thermally and actively driven, the latter caused by forces from chromatin and cytoskeleton. Such undulations might affect gene regulation as well as contribute to the anomalously high rates of nuclear transport by, e.g., stirring of molecules next to NE, or increasing flux of molecules through the nuclear pores.
Keywords: active materials; chromatin; nuclear envelope; nuclear lamina.
Conflict of interest statement
The authors declare no conflict of interest.
Figures










Similar articles
-
[Karyoskeletal and nuclear envelope proteins in cell cycle progression--known proteins in new functions].Postepy Biochem. 2010;56(4):362-72. Postepy Biochem. 2010. PMID: 21473040 Review. Polish.
-
The Dynamic Nature of the Nuclear Envelope.Curr Biol. 2018 Apr 23;28(8):R487-R497. doi: 10.1016/j.cub.2018.01.073. Curr Biol. 2018. PMID: 29689232 Review.
-
Pushing the envelope: structure, function, and dynamics of the nuclear periphery.Annu Rev Cell Dev Biol. 2005;21:347-80. doi: 10.1146/annurev.cellbio.21.090704.151152. Annu Rev Cell Dev Biol. 2005. PMID: 16212499 Review.
-
Cell-cycle-dependent dynamics of nuclear pores: pore-free islands and lamins.J Cell Sci. 2006 Nov 1;119(Pt 21):4442-51. doi: 10.1242/jcs.03207. J Cell Sci. 2006. PMID: 17074834
-
Shaping the endoplasmic reticulum into the nuclear envelope.J Cell Sci. 2008 Jan 15;121(Pt 2):137-42. doi: 10.1242/jcs.005777. J Cell Sci. 2008. PMID: 18187447
Cited by
-
The role of vimentin-nuclear interactions in persistent cell motility through confined spaces.New J Phys. 2021 Sep;23:093042. doi: 10.1088/1367-2630/ac2550. Epub 2021 Sep 29. New J Phys. 2021. PMID: 35530563 Free PMC article.
-
Lipid droplets are intracellular mechanical stressors that impair hepatocyte function.Proc Natl Acad Sci U S A. 2023 Apr 18;120(16):e2216811120. doi: 10.1073/pnas.2216811120. Epub 2023 Apr 10. Proc Natl Acad Sci U S A. 2023. PMID: 37036981 Free PMC article.
-
Curvature fluctuations of fluid vesicles reveal hydrodynamic dissipation within the bilayer.Proc Natl Acad Sci U S A. 2024 Oct 29;121(44):e2413557121. doi: 10.1073/pnas.2413557121. Epub 2024 Oct 23. Proc Natl Acad Sci U S A. 2024. PMID: 39441635 Free PMC article.
-
Surface Fluctuations and Coalescence of Nucleolar Droplets in the Human Cell Nucleus.Phys Rev Lett. 2018 Oct 5;121(14):148101. doi: 10.1103/PhysRevLett.121.148101. Phys Rev Lett. 2018. PMID: 30339413 Free PMC article.
-
Nuclear Morphological Remodeling in Human Granulocytes Is Linked to Prenylation Independently from Cytoskeleton.Cells. 2020 Nov 20;9(11):2509. doi: 10.3390/cells9112509. Cells. 2020. PMID: 33233551 Free PMC article.
References
-
- Steen H, Lindmo T. Cellular and nuclear volume during the cell cycle of nihk 3025 cells. Cell Tissue Kinet. 1978;11:69–81. - PubMed
-
- Fidorra J, Mielke T, Booz J, Feinendegen L. Cellular and nuclear volume of human cells during the cell cycle. Radiat Environ Biophys. 1981;19:205–214. - PubMed
-
- Alberts B, et al. Molecular Biology of the Cell. Garland Sci; New York: 2014.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

