The metal chaperone Atox1 regulates the activity of the human copper transporter ATP7B by modulating domain dynamics
- PMID: 28900031
- PMCID: PMC5672040
- DOI: 10.1074/jbc.M117.811752
The metal chaperone Atox1 regulates the activity of the human copper transporter ATP7B by modulating domain dynamics
Abstract
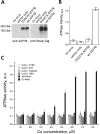
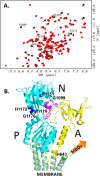
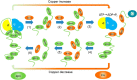
The human transporter ATP7B delivers copper to the biosynthetic pathways and maintains copper homeostasis in the liver. Mutations in ATP7B cause the potentially fatal hepatoneurological disorder Wilson disease. The activity and intracellular localization of ATP7B are regulated by copper, but the molecular mechanism of this regulation is largely unknown. We show that the copper chaperone Atox1, which delivers copper to ATP7B, and the group of the first three metal-binding domains (MBD1-3) are central to the activity regulation of ATP7B. Atox1-Cu binding to ATP7B changes domain dynamics and interactions within the MBD1-3 group and activates ATP hydrolysis. To understand the mechanism linking Atox1-MBD interactions and enzyme activity, we have determined the MBD1-3 conformational space using small angle X-ray scattering and identified changes in MBD dynamics caused by apo-Atox1 and Atox1-Cu by solution NMR. The results show that copper transfer from Atox1 decreases domain interactions within the MBD1-3 group and increases the mobility of the individual domains. The N-terminal segment of MBD1-3 was found to interact with the nucleotide-binding domain of ATP7B, thus physically coupling the domains involved in copper binding and those involved in ATP hydrolysis. Taken together, the data suggest a regulatory mechanism in which Atox1-mediated copper transfer activates ATP7B by releasing inhibitory constraints through increased freedom of MBD1-3 motions.
Keywords: ATPase; copper transport; membrane protein; membrane transport; nuclear magnetic resonance (NMR); protein NMR; protein dynamic.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures


References
-
- Lutsenko S., Barnes N. L., Bartee M. Y., and Dmitriev O. Y. (2007) Function and regulation of human copper-transporting ATPases. Physiol. Rev. 87, 1011–1046 - PubMed
-
- Dmitriev O. Y. (2011) Mechanism of tumor resistance to cisplatin mediated by the copper transporter ATP7B. Biochem. Cell Biol. 89, 138–147 - PubMed
-
- Kuo M. T., Chen H. H., Song I. S., Savaraj N., and Ishikawa T. (2007) The roles of copper transporters in cisplatin resistance. Cancer Metastasis Rev. 26, 71–83 - PubMed
-
- Li X. P., Yin J. Y., Wang Y., He H., Li X., Gong W. J., Chen J., Qian C. Y., Zheng Y., Li F., Yin T., Gong Z. C., Zhou B. T., Zhang Y., Xiao L., et al. (2014) The ATP7B genetic polymorphisms predict clinical outcome to platinum-based chemotherapy in lung cancer patients. Tumour Biol. 35, 8259–8265 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

