Impaired Cerebellum to Primary Motor Cortex Associative Plasticity in Parkinson's Disease and Spinocerebellar Ataxia Type 3
- PMID: 28900413
- PMCID: PMC5581840
- DOI: 10.3389/fneur.2017.00445
Impaired Cerebellum to Primary Motor Cortex Associative Plasticity in Parkinson's Disease and Spinocerebellar Ataxia Type 3
Abstract
Background: Functional perturbation of the cerebellum (CB)-motor cortex (M1) interactions may underlie pathophysiology of movement disorders, such as Parkinson's disease (PD) and spinocerebellar ataxia type 3 (SCA3). Recently, M1 motor excitability can be bidirectionally modulated in young subjects by corticocortical paired associative stimulation (PAS) on CB and contralateral M1 with transcranial magnetic stimulation (TMS), probably through the cerebello-dentato-thalamo-cortical (CDTC) circuit. In this study, we investigated the CB to M1-associative plasticity in healthy elderly PD and SCA3.
Methods: Ten right-handed PD patients, nine gene-confirmed SCA3 patients, and 10 age-matched healthy controls (HC) were studied. One hundred and twenty pairs of TMS of the left M1 preceded by right lateral CB TMS at an interstimulus interval of 2 (CB → M1 PAS2ms) and 6 ms (CB → M1 PAS6ms) were, respectively, applied with at least 1-week interval. M1 excitability was assessed by motor-evoked potential (MEP) amplitude, short-interval intracortical inhibition (SICI), intracortical facilitation (ICF), and cerebellar inhibition (CBI) at the first dorsal interosseous muscle of the right hand before and after the CB → M1 PAS.
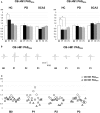
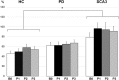
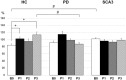
Results: The M1 excitability represented by MEP amplitude was significantly facilitated and suppressed in the HC group by CB → M1 PAS2ms and CB → M1 PAS6ms, respectively. The bidirectional modulation on MEP amplitude was absent in the PD and SCA3 groups. SICI and the baseline CBI were significantly reduced in the SCA3 group compared to those of the HC group irrespective of the CB → M1 PAS protocols. There was a significant reduction of CBI immediately and 60 min after the CB → M1 PAS protocols in the HC group but not in the patient groups. No significant change of ICF was found.
Conclusion: Corticocortical CB → M1 PAS can induce bidirectional motor cortical plasticity in M1 for healthy aged subjects. The modulation may be independent of the inhibitory neurocircuits, such as SICI and CBI, and the facilitatory mechanism like ICF. Both patients with PD and SCA3 showed impairment of such plasticity, suggesting significant functional perturbation of the CDTC circuit.
Keywords: Parkinson’s disease; cerebellar inhibition; motor cortex; paired associative stimulation; spinocerebellar ataxia type 3.
Figures
References
-
- Allen GI, Tsukahara N. Cerebrocerebellar communication systems. Physiol Rev (1974) 54(4):957–1006. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

