A Nanosystem of Amphiphilic Oligopeptide-Drug Conjugate Actualizing Both αvβ3 Targeting and Reduction-Triggered Release for Maytansinoid
- PMID: 28900511
- PMCID: PMC5595133
- DOI: 10.7150/thno.20242
A Nanosystem of Amphiphilic Oligopeptide-Drug Conjugate Actualizing Both αvβ3 Targeting and Reduction-Triggered Release for Maytansinoid
Abstract
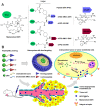
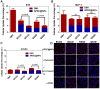
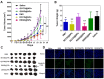
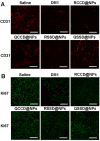
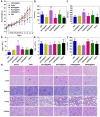
To design a prodrug-based self-assembling nanosystem with both ligand targeting and stimuli-responsive features, and elucidate the superiority of each targeting strategy and the synergistic effect between them, we synthesized four small molecule amphiphilic peptide-drug conjugates (APDCs) using maytansinoid (DM1) as a cytotoxic agent, cRGDfK as a homing peptide, and disulfide (SS) or thioether (SMCC) as linker. Owing to their amphiphilicity, the APDCs could self-assemble into nanoparticles (APDC@NPs) which were evaluated in vitro in three different cell lines and in vivo in tumor-bearing C57BL/6 mice. The RSSD@NPs showed the strongest interaction with αvβ3 integrin, highest cell uptake and intracellular free drug level, and best antitumor efficacy in vitro and in vivo, while it shared the same goodness with other test nanosystems in terms of high drug loading, EPR effect and free of potentially toxic polymers. Especially, the in vivo efficacy of RSSD@NPs was 2 fold of free DM1 which is too cytotoxic to be a drug, while the active targeted APDC@NPs demonstrated acceptable system, tissue and blood compatibility. In αvβ3-positive cells or tumors, the RGD targeting contributed much more than disulfide in anticancer effect. The maximum synergism of the two strategies reached to 22 fold in vitro and 3 fold in vivo. Generally, the active targeting, prodrug and nanosystem could significantly decrease the toxicity of free DM1 and improve its therapy outcome via combining active targeting, prodrug and nanopreparation, especially the dual targeting strategies and their synergism.
Keywords: Amphiphilic peptide-drug conjugate; antitumor therapy.; endocytosis; maytansinoid DM1; reduction-triggered drug release; αvβ3-targeted nanoparticles.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures


References
-
- Choi KY, Yoon HY, Kim JH. et al. Smart nanocarrier based on PEGylated hyaluronic acid for cancer therapy. ACS Nano. 2011;5(11):8591–8599. - PubMed
-
- Mikhaylov G, Mikac U, Magaeva AA. et al. Ferri-liposomes as an MRI-visible drug-delivery system for targeting tumours and their microenvironment. Nat Nanotech. 2011;6(9):594–602. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

