Resveratrol-Enriched Rice Attenuates UVB-ROS-Induced Skin Aging via Downregulation of Inflammatory Cascades
- PMID: 28900534
- PMCID: PMC5576414
- DOI: 10.1155/2017/8379539
Resveratrol-Enriched Rice Attenuates UVB-ROS-Induced Skin Aging via Downregulation of Inflammatory Cascades
Erratum in
-
Corrigendum to "Resveratrol-Enriched Rice Attenuates UVB-ROS-Induced Skin Aging via Downregulation of Inflammatory Cascades".Oxid Med Cell Longev. 2018 Feb 20;2018:6052623. doi: 10.1155/2018/6052623. eCollection 2018. Oxid Med Cell Longev. 2018. PMID: 29675135 Free PMC article.
Abstract
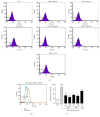
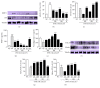
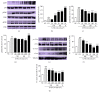
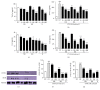
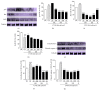
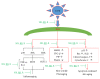
The skin is the outermost protective barrier between the internal and external environments in humans. Chronic exposure to ultraviolet (UV) radiation is a major cause of skin aging. UVB radiation penetrates the skin and induces ROS production that activates three major skin aging cascades: matrix metalloproteinase- (MMP-) 1-mediated aging; MAPK-AP-1/NF-κB-TNF-α/IL-6, iNOS, and COX-2-mediated inflammation-induced aging; and p53-Bax-cleaved caspase-3-cytochrome C-mediated apoptosis-induced aging. These mechanisms are collectively responsible for the wrinkling and photoaging characteristic of UVB-induced skin aging. There is an urgent requirement for a treatment that not only controls these pathways to prevent skin aging but also avoids the adverse effects often encountered when applying bioactive compounds in concentrated doses. In this study, we investigated the efficacy of genetically modified normal edible rice (NR) that produces the antiaging compound resveratrol (R) as a treatment for skin aging. This resveratrol-enriched rice (RR) overcomes the drawbacks of R and enhances its antiaging potential by controlling the abovementioned three major pathways of skin aging. RR does not exhibit the toxicity of R alone and promisingly downregulates the pathways underlying UVB-ROS-induced skin aging. These findings advocate the use of RR as a nutraceutical for antiaging purposes.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

