Clinical outcomes in ER+ HER2 -node-positive breast cancer patients who were treated according to the Recurrence Score results: evidence from a large prospectively designed registry
- PMID: 28900632
- PMCID: PMC5591314
- DOI: 10.1038/s41523-017-0033-7
Clinical outcomes in ER+ HER2 -node-positive breast cancer patients who were treated according to the Recurrence Score results: evidence from a large prospectively designed registry
Abstract
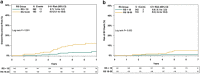
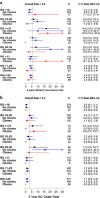
The Recurrence Score® is increasingly used in node-positive ER+ HER2-negative breast cancer. This retrospective analysis of a prospectively designed registry evaluated treatments/outcomes in node-positive breast cancer patients who were Recurrence Score-tested through Clalit Health Services from 1/2006 through 12/2011 (N = 709). Medical records were reviewed to verify treatments/recurrences/survival. Median follow-up, 5.9 years; median age, 62 years; 53.9% grade 2; 69.8% tumors ≤ 2 cm; 84.5% invasive ductal carcinoma; 42.0% N1mi, and 37.2%/15.5%/5.2% with 1/2/3 positive nodes; 53.4% Recurrence Score < 18, 36.4% Recurrence Score 18-30, and 10.2% Recurrence Score ≥ 31. Overall, 26.9% received adjuvant chemotherapy: 7.1%, 39.5%, and 86.1% in the Recurrence Score < 18, 18-30, and ≥ 31 group, respectively. The 5-year Kaplan-Meier estimates for distant recurrence were 3.2%, 6.3%, and 16.9% for these respective groups and the corresponding 5-year breast cancer death estimates were 0.5%, 3.4%, and 5.7%. In Recurrence Score < 18 patients, 5-year distant-recurrence rates for N1mi/1 positive node/2-3 positive nodes were 1.2%/4.4%/5.4%. As patients were not randomized to treatment and treatment decision is heavily influenced by Recurrence Score, analysis of 5-year distant recurrence by chemotherapy use was exploratory and should be interpreted cautiously: In Recurrence Score < 18, recurrence rate was 7.7% in chemotherapy-treated (n = 27) and 2.9% in chemotherapy-untreated patients (n = 352); P = 0.245. In Recurrence Score 18-30, recurrence rate in chemotherapy-treated patients (n = 102) was significantly lower than in untreated patients (n = 156) (1.0% vs. 9.7% P = 0.019); in Recurrence Score ≤ 25 (the RxPONDER study cutoff), recurrence rate was 2.3% in chemotherapy-treated (n = 89) and 4.4% in chemotherapy-untreated patients (n = 488); P = 0.521. In conclusion, our findings support using endocrine therapy alone in ER+ HER2-negative breast cancer patients with micrometastases/1-3 positive nodes and Recurrence Score < 18.
Conflict of interest statement
Salomon M. Stemmer reports receiving grant funding from Teva; receiving travel expenses from Genomic Health, Inc; and conducting research funded by Teva. Lior Soussan-Gutman reports being employed by and holding stock options in Teva Pharmaceutical Industries Ltd. Avital Bareket-Samish reports being a consultant/medical writer for Teva Pharmaceutical Industries Ltd, Genomic Health Inc (including for the purpose of the current analysis), Bayer, and Roche. Christer Svedman, Debbie McCullough, and Steven Shak report being employed by Genomic Health and having stock ownership in Genomic Health. Christer Svedman, and Steven Shak report having intellectual property interest in Genomic Health. Steven Shak reports having a leadership role at Genomic Health. Noa Ben-Baruch reports receiving honoraria from Teva, and serving on the speaker’s bureau of Genomic Health. Marian Steiner, Shulamith Rizel, David B Geffen, Bella Nisenbaum, Tamar Peretz, Kevin Isaacs, Ora Rosengarten, Georgeta Fried, and Nicky Liebermann declare no competing financial interests.
Figures


References
-
- NCCN Clinical Practice Guidelines in Oncology. Breast Cancer. Version 2.2016. Available at: http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed Jan 1, 2017.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

