Pharmacological and mechanical interventions for labour induction in outpatient settings
- PMID: 28901007
- PMCID: PMC6483740
- DOI: 10.1002/14651858.CD007701.pub3
Pharmacological and mechanical interventions for labour induction in outpatient settings
Abstract
Background: Induction of labour is carried out for a variety of indications and using a range of methods. For women at low risk of pregnancy complications, some methods of induction of labour or cervical ripening may be suitable for use in outpatient settings.
Objectives: To examine pharmacological and mechanical interventions to induce labour or ripen the cervix in outpatient settings in terms of effectiveness, maternal satisfaction, healthcare costs and, where information is available, safety.
Search methods: We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (30 November 2016) and reference lists of retrieved studies.
Selection criteria: We included randomised controlled trials examining outpatient cervical ripening or induction of labour with pharmacological agents or mechanical methods. Cluster trials were eligible for inclusion.
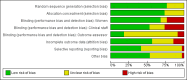
Data collection and analysis: Two review authors independently assessed trials for inclusion and risk of bias, extracted data and checked them for accuracy. We assessed evidence using the GRADE approach.
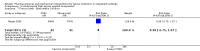
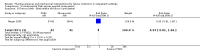
Main results: This updated review included 34 studies of 11 different methods for labour induction with 5003 randomised women, where women received treatment at home or were sent home after initial treatment and monitoring in hospital.Studies examined vaginal and intracervical prostaglandin E₂ (PGE₂), vaginal and oral misoprostol, isosorbide mononitrate, mifepristone, oestrogens, amniotomy and acupuncture, compared with placebo, no treatment, or routine care. Trials generally recruited healthy women with a term pregnancy. The risk of bias was mostly low or unclear, however, in 16 trials blinding was unclear or not attempted. In general, limited data were available on the review's main and additional outcomes. Evidence was graded low to moderate quality. 1. Vaginal PGE₂ versus expectant management or placebo (5 studies)Fewer women in the vaginal PGE₂ group needed additional induction agents to induce labour, however, confidence intervals were wide (risk ratio (RR) 0.52, 95% confidence interval (CI) 0.27 to 0.99; 150 women; 2 trials). There were no clear differences between groups in uterine hyperstimulation (with or without fetal heart rate (FHR) changes) (RR 3.76, 95% CI 0.64 to 22.24; 244 women; 4 studies; low-quality evidence), caesarean section (RR 0.80, 95% CI 0.49 to 1.31; 288 women; 4 studies; low-quality evidence), or admission to a neonatal intensive care unit (NICU) (RR 0.32, 95% CI 0.10 to 1.03; 230 infants; 3 studies; low-quality evidence).There was no information on vaginal birth within 24, 48 or 72 hours, length of hospital stay, use of emergency services or maternal or caregiver satisfaction. Serious maternal and neonatal morbidity or deaths were not reported. 2. Intracervical PGE₂ versus expectant management or placebo (7 studies) There was no clear difference between women receiving intracervical PGE₂ and no treatment or placebo in terms of need for additional induction agents (RR 0.98, 95% CI 0.74 to 1.32; 445 women; 3 studies), vaginal birth not achieved within 48 to 72 hours (RR 0.83, 95% CI 0.68 to 1.02; 43 women; 1 study; low-quality evidence), uterine hyperstimulation (with FHR changes) (RR 2.66, 95% CI 0.63 to 11.25; 488 women; 4 studies; low-quality evidence), caesarean section (RR 0.90, 95% CI 0.72 to 1.12; 674 women; 7 studies; moderate-quality evidence), or babies admitted to NICU (RR 1.61, 95% CI 0.43 to 6.05; 215 infants; 3 studies; low-quality evidence). There were no uterine ruptures in either the PGE₂ group or placebo group.There was no information on vaginal birth not achieved within 24 hours, length of hospital stay, use of emergency services, mother or caregiver satisfaction, or serious morbidity or neonatal morbidity or perinatal death. 3. Vaginal misoprostol versus placebo (4 studies)One small study reported on the rate of perinatal death with no clear differences between groups; there were no deaths in the treatment group compared with one stillbirth (reason not reported) in the control group (RR 0.34, 95% CI 0.01 to 8.14; 77 infants; 1 study; low-quality evidence).There was no clear difference between groups in rates of uterine hyperstimulation with FHR changes (RR 1.97, 95% CI 0.43 to 9.00; 265 women; 3 studies; low-quality evidence), caesarean section (RR 0.94, 95% CI 0.61 to 1.46; 325 women; 4 studies; low-quality evidence), and babies admitted to NICU (RR 0.89, 95% CI 0.54 to 1.47; 325 infants; 4 studies; low-quality evidence).There was no information on vaginal birth not achieved within 24, 48 or 72 hours, additional induction agents required, length of hospital stay, use of emergency services, mother or caregiver satisfaction, serious maternal, and other neonatal, morbidity or death.No substantive differences were found for other comparisons. One small study found that women who received oral misoprostol were more likely to give birth within 24 hours (RR 0.65, 95% CI 0.48 to 0.86; 87 women; 1 study) and were less likely to require additional induction agents (RR 0.60, 95% CI 0.37 to 0.97; 127 women; 2 studies). Women who received mifepristone were also less likely to require additional induction agents (average RR 0.59, 95% CI 0.37 to 0.95; 311 women; 4 studies; I² = 74%); however, this result should be interpreted with caution due to high heterogeneity. One trial each of acupuncture and outpatient amniotomy were included, but few review outcomes were reported.
Authors' conclusions: Induction of labour in outpatient settings appears feasible and important adverse events seem rare, however, in general there is insufficient evidence to detect differences. There was no strong evidence that agents used to induce labour in outpatient settings had an impact (positive or negative) on maternal or neonatal health. There was some evidence that compared to placebo or no treatment, induction agents administered on an outpatient basis reduced the need for further interventions to induce labour, and shortened the interval from intervention to birth.We do not have sufficient evidence to know which induction methods are preferred by women, the interventions that are most effective and safe to use in outpatient settings, or their cost effectiveness. Further studies where various women-friendly outpatient protocols are compared head-to-head are required. As part of such work, women should be consulted on what sort of management they would prefer.
Conflict of interest statement
Joshua P Vogel: none known.
Alfred O Osoti: none known.
Anthony J Kelly: none known.
Stefania Livio: none known.
Jane E Norman: Jane Norman was an investigator on two trials included in this review (Bollapragada 2006b; Osman 2006); the reports from these trials were independently assessed by two other review authors. Jane Norman has received a grant of GBP 11,000 (paid to her institution) from the Chief Scientist's Office, Scottish Executive, for an epidemiological study entitled: "Ferguson EF, Norman JE, Chalmers J, Shanks E, Finlayson A. Investigation of the beneficial and adverse effects of induction of labour." Jane Norman has received a number of research grants (paid to her institution) to support research into improving perinatal outcome ‐ none specifically related to immediate versus deferred delivery. Jane has also received small amounts of money for speaking at meetings about prematurity but not immediate versus deferred delivery.
Zarko Alfirevic: none known.
Figures




















































































































































Update of
-
Different methods for the induction of labour in outpatient settings.Cochrane Database Syst Rev. 2010 Aug 4;(8):CD007701. doi: 10.1002/14651858.CD007701.pub2. Cochrane Database Syst Rev. 2010. Update in: Cochrane Database Syst Rev. 2017 Sep 13;9:CD007701. doi: 10.1002/14651858.CD007701.pub3. PMID: 20687092 Free PMC article. Updated.
References
References to studies included in this review
Agarwal 2012 {published data only}
-
- Agarwal K, Batra A, Dabral A, Aggarwal A. Evaluation of isosorbide mononitrate for cervical ripening prior to induction of labor for postdated pregnancy in an outpatient setting. International Journal of Gynecology and Obstetrics 2012;118(3):205‐9. - PubMed
Attanayake 2014 {published data only}
-
- Attanayake K, Goonewardene M. Outpatient cervical ripening with vaginal isosorbide mononitrate in uncomplicated singleton pregnancies at 39 weeks gestation: A double blind randomized controlled trial. Sri Lanka Journal of Obstetrics and Gynaecology 2014;36(Suppl 1):28, Abstract no: FC 10.5. - PubMed
Bollapragada 2006a {published data only}
-
- Bollapragada S, Mackenzie F, Norrie J, Petrou S, Reid M, Greer I, et al. IMOP: randomised placebo controlled trial of outpatient cervical ripening with isosorbide mononitrate (IMN) prior to induction of labour ‐ clinical trial with analyses of efficacy, cost effectiveness and acceptability. BMC Pregnancy and Childbirth 2006;6:25. [DOI: 10.1186/1471-2393-6-25] - DOI - PMC - PubMed
-
- Bollapragada SS, MacKenzie F, Norrie J, Petrou S, Reid M, Greer IA, et al. Randomized placebo controlled trial of outpatient cervical ripening with isosorbide mononitrate (IMN) prior to induction of labour ‐ clinical trial with analyses of efficacy, cost effectiveness and acceptability. The IMOP study [abstract]. Journal of Obstetrics and Gynaecology 2007;27(Suppl 1):S22. - PubMed
-
- Bollapragada SS, MacKenzie F, Norrie JD, Eddama O, Petrou S, Reid M, et al. Randomised placebo‐controlled trial of outpatient (at home) cervical ripening with isosorbide mononitrate (IMN) prior to induction of labour‐clinical trial with analyses of efficacy and acceptability. The IMOP study. BJOG: an international journal of obstetrics and gynaecology 2009;116(9):1185‐95. - PubMed
-
- Eddama O, Petrou S, Schroeder L, Bollapragada SS, Mackenzie F, Norrie J, et al. The cost‐effectiveness of outpatient (at home) cervical ripening with isosorbide mononitrate prior to induction of labour. BJOG: an international journal of obstetrics and gynaecology 2009;116(9):1196‐203. - PubMed
-
- Reid M, Lorimer K, Norman JE, Bollapragada SS, Norrie J. The home as an appropriate setting for women undertaking cervical ripening before the induction of labour. Midwifery 2011;27(1):30‐5. - PubMed
Bullarbo 2007 {published data only}
-
- Bullarbo M, Orrskog ME, Andersch B, Granström L, Norström A, Ekerhovd E. Outpatient vaginal administration of the nitric oxide donor isosorbide mononitrate for cervical ripening and labor induction postterm: a randomized controlled study. American Journal of Obstetrics and Gynecology 2007;196(1):50.e1‐5. - PubMed
Buttino 1990 {published data only}
-
- Buttino LT Jr, Garite TJ. Intracervical prostaglandin in postdate pregnancy. A randomized trial. Journal of Reproductive Medicine 1990;35(2):155‐8. - PubMed
Elliott 1998 {published data only}
-
- Elliot CL, Brennand JE, Calder AA. The effect of mifepristone (RU486) on cervical ripening and induction of labour in human pregnancy. 27th British Congress of Obstetrics and Gynaecology; 1995 July 4‐7; Dublin, Ireland. 1995:207.
-
- Elliott CL, Brennand JE, Calder AA. The effects of mifepristone on cervical ripening and labor induction in primigravidae. Obstetrics and Gynecology 1998;92(5):804‐9. - PubMed
Frydman 1992 {published data only}
-
- Frydman R, Baton C, Lelaidier C, Vial M, Bourget P, Fernandez H. Mifepristone for induction of labour. Lancet 1991;337(8739):488‐9. - PubMed
-
- Frydman R, Lelaidier C, Baton‐Saint‐Mleux C, Fernandez H, Vial M, Bourget P. Labor induction in women at term with mifepristone (RU 486): a double blind, randomized, placebo‐controlled study. Obstetrics and Gynecology 1992;80(6):972‐5. - PubMed
-
- Frydman R, Lelaidier C, Baton‐Saint‐Mleux C, Fernandez H, Vial M, Bourget P. Labor induction in women at term with mifepristone (RU 486): a double‐blind, randomized, placebo‐controlled study. International Journal of Gynecology and Obstetrics 1993;42(2):220. - PubMed
-
- Frydman R, Taylor S, Paoli C, Pourade A. RU 486 (mifepristone): A new tool for labour induction in term women with fetus alive [Le RU 486 (mifepristone) un novel outil pour le declenchment du travail a terme]. Contraception, Fertilite, Sexualite 1992;20(12):1133‐6. - PubMed
-
- Lelaidier C, Benifla JL, Fernandez H, Baton C, Bourget P, Bourrier MC, et al. RU 486 (mifepristone) in medical indications for labour induction in pregnancies at term: results of a randomized, double‐blind study of RU 486 vs placebo. Journal de Gynecologie, Obstetrique et Biologie de la Reproduction 1993;22:92‐100. - PubMed
Gaffaney 2009 {published data only}
-
- Gaffaney CA, Saul LL, Rumney PJ, Morrison EH, Thomas S, Nageotte MP, et al. Outpatient oral misoprostol for prolonged pregnancies: a pilot investigation. American Journal of Perinatology 2009;26(9):673‐7. - PubMed
Ghanaie 2013 {published data only}
-
- Ghanaie MM, Mirblouk F, Godarzi R, Shakiba M. Effect of outpatient isorbide mononitrate on success of labor induction. Journal of Babol University of Medical Sciences 2013;15(2):12‐7.
Giacalone 1998 {published data only}
-
- Giacalone PL, Targosz V, Laffargue F, Boog G, Faure JM. Cervical ripening with mifepristone before labor induction: a randomized study. Obstetrics and Gynecology 1998;92(4 Pt 1):487‐92. - PubMed
Gittens 1996 {published data only}
-
- Gittens L, Schenkel C, Strassberg S, Apuzzio J. Vaginal birth after cesarean section: comparison of outpatient use of prostaglandin gel to expectant management. American Journal of Obstetrics and Gynecology 1996;174(1 Pt 2):354.
Habib 2008 {published data only}
-
- Habib SM, Emam SS, Saber AS. Outpatient cervical ripening with nitric oxide donor isosorbide mononitrate prior to induction of labor. International Journal of Gynaecology and Obstetrics 2008;101(1):57‐61. - PubMed
Hage 1993 {published data only}
-
- Hage P, Shaw J, Zarou D, Fleisher J, Wehbeh H. Double blind randomized trial to evaluate the role of outpatient use of PGE2 in cervical ripening. American Journal of Obstetrics and Gynecology 1993;168(1 Part 2):430.
Harper 2006 {published data only}
-
- Harper TC, Coeytaux RR, Chen W, Campbell K, Kaufman JS, Moise KJ, et al. A randomized controlled trial of acupuncture for initiation of labor in nulliparous women. Journal of Maternal‐Fetal and Neonatal Medicine 2006;19(8):465‐70. - PubMed
Incerpi 2001 {published data only}
-
- Incerpi M, Fassett M, Kjos S, Tran S, Wing D. Vaginally administered misoprostol for outpatient labor induction in pregnancies with diabetes mellitus [abstract]. American Journal of Obstetrics and Gynecology 2001;184(1):S120. - PubMed
-
- Incerpi MH, Fassett MJ, Kjos SL, Tran SH, Wing DA. Vaginally administered misoprostol for outpatient cervical ripening in pregnancies complicated by diabetes mellitus. American Journal of Obstetrics and Gynecology 2001;185(4):916‐9. - PubMed
Kipikasa 2005 {published data only}
-
- Kipikasa JH, Adair CD, Williamson J, Breen JM, Medford LK, Sanchez‐Ramos L. Use of misoprostol on an outpatient basis for postdate pregnancy. International Journal of Gynaecology and Obstetrics 2005;88(2):108‐11. - PubMed
Larmon 2002 {published data only}
-
- Larmon JE, Magann EF, Dickerson GA, Morrison JC. Outpatient cervical ripening with prostaglandin E2 and estradiol. Journal of Maternal‐Fetal and Neonatal Medicine 2002;11(2):113‐7. - PubMed
Lelaidier 1994 {published data only}
-
- Lelaidier C, Baton C, Benifla JL, Fernandez H, Bourget P, Frydman R. Mifepristone for labour induction after previous caesarean section. British Journal of Obstetrics and Gynaecology 1994;101(6):501‐3. - PubMed
Lien 1998 {published data only}
-
- Lien JM, Morgan MA, Garite TJ, Kennedy KA, Sassoon DA, Freeman RK. Antepartum cervical ripening: applying prostaglandin e2 gel in conjunction with scheduled nonstress tests in postdate pregnancies. American Journal of Obstetrics and Gynecology 1998;179(2):453‐8. - PubMed
Lyons 2001 {published data only}
-
- Lyons C, Rumney P, Huang W, Morrison E, Thomas S, Nageotte M, et al. Outpatient cervical ripening with oral misoprostol post‐term: induction rates decreased. American Journal of Obstetrics and Gynecology 2001;184(1):S116.
Magann 1998 {published data only}
-
- Magann E, Chauhan SP, Nevils BG, McNamara MF, Kinsella MJ, Morrison JC. Management of pregnancies beyond forty‐one weeks' gestation with an unfavorable cervix. American Journal of Obstetrics and Gynecology 1998;178(6):1279‐87. - PubMed
McKenna 1999 {published data only}
-
- McKenna DS, Costa SW, Samuels P. Prostaglandin E2 cervical ripening without subsequent induction of labor. Obstetrics and Gynecology 1999;94(1):11‐4. - PubMed
McKenna 2004 {published data only}
-
- McKenna DS, Ester JB, Proffitt M, Waddell KR. Misoprostol outpatient cervical ripening without subsequent induction of labor: a randomized trial. Obstetrics and Gynecology 2004;104(3):579‐84. - PubMed
Meyer 2005 {published data only}
-
- Meyer M, Pflum J. Outpatient administration of misoprostol decreases induction time. American Journal of Obstetrics and Gynecology 2002;187(6 Pt 2):S167.
-
- Meyer M, Pflum J, Howard D. Outpatient misoprostol compared with dinoprostone gel for preinduction cervical ripening: a randomized controlled trial. Obstetrics and Gynecology 2005;105(3):466‐72. - PubMed
Newman 1997 {published data only}
-
- Newman M, Newman R. Multiple‐dose PGE2 cervical ripening on an outpatient basis: safety and efficacy. American Journal of Obstetrics and Gynecology 1997;176(1 Pt 2):S112.
O'Brien 1995 {published data only}
-
- O'Brien JM, Mercer B, Cleary N, Sibai BM. Efficacy of outpatient induction with low dose intravaginal prostaglandin E2: A randomized, double‐blind, placebo‐controlled trial. American Journal of Obstetrics and Gynecology 1995;172:424. - PubMed
-
- O'Brien JM, Mercer BM, Cleary NT, Sibai BM. Efficacy of outpatient induction with low‐dose intravaginal prostaglandin E2: A randomized, double blind, placebo‐controlled trial. American Journal of Obstetrics and Gynecology 1995;173(6):1855‐9. - PubMed
Oboro 2005 {published data only}
-
- Oboro VO, Tabowei TO. Outpatient misoprostol cervical ripening without subsequent induction of labor to prevent post‐term pregnancy. Acta Obstetricia et Gynecologica Scandinavica 2005;84(7):628‐31. - PubMed
Rayburn 1999 {published data only}
-
- Rayburn W, Lucas M, Gittens L, Goodwin TM, Baxi L, Gall S, et al. Attempted vaginal birth after cesarean section: a multicenter comparison of outpatient prostaglandin E2 gel with expectant management. Primary Care Update for Ob/Gyns 1998;5(4):182‐3. - PubMed
-
- Rayburn WF, Gittens LN, Lucas MJ, Gall SA, Martin ME. Weekly administration of prostaglandin e2 gel compared with expectant management in women with previous cesareans Prepidil gel study group. Obstetrics and Gynecology 1999;94(2):250‐4. - PubMed
Rijnders 2011 {published data only}
-
- ISRCTN47736435. Costs and effects of amniotomy at home for induction of post term pregnancy. isrctn.com/ISRCTN47736435 (first received 9 January 2006).
-
- Rijnders MEB. A randomised controlled trial of amniotomy at home for induction between 292 and 294 days gestation. Interventions in Midwife Led Care in the Netherlands to Achieve Optimal Birth Outcomes: Effects and Women's Experiences. Amsterdam: University of Amsterdam, 2011.
Sawai 1991 {published data only}
-
- Sawai SK, Williams MC, O'Brien WF, Angel JL, Mastrogiannis DS, Johnson L. Sequential outpatient application of intravaginal prostaglandin E2 gel in the management of postdates pregnancies. Obstetrics and Gynecology 1991;78(1):19‐23. - PubMed
-
- Williams MG, O'Brien WF, Sawai SK, Knuppel RA. Outpatient cervical ripening in the postdates pregnancy. Proceedings of 10th Annual Meeting of Society of Perinatal Obstetricians; 1990 Jan 23‐27; Houston, Texas, USA. 1990:533.
Sawai 1994 {published data only}
-
- Sawai SK, O'Brien WF, Mastrogiannis DS, Krammer J, Mastry MG, Porter GW. Patient‐administered outpatient intravaginal prostaglandin E2 suppositories in post‐date pregnancies: a double‐blind, randomized, placebo‐controlled study. Obstetrics and Gynecology 1994;84(5):807‐10. - PubMed
-
- Sawai SK, O'Brien WF, Mastrogiannis MS, Mastry MG, Porter GW, Johnson L. Outpatient prostaglandin E2 suppositories in postdates pregnancies. American Journal of Obstetrics and Gynecology 1992;166(1 Pt 2):400.
Schmitz 2014 {published data only}
-
- Schmitz T, Closset E, Fuchs F, Maillard F, Rozenberg P, Anselem O, et al. Outpatient cervical ripening with nitric oxide (NO) donors for prolonged pregnancy in nullipara: the NOCETER randomized, multicentre, double‐blind, placebo‐controlled trial. American Journal of Obstetrics and Gynecology 2014;210(1 Suppl):S19.
-
- Schmitz T, Fuchs F, Closset E, Rozenberg P, Winer N, Perrotin F, et al. Outpatient cervical ripening by nitric oxide donors for prolonged pregnancy: a randomized controlled trial. Obstetrics and Gynecology 2014;124(6):1089‐97. - PubMed
Stenlund 1999 {published data only}
-
- Stenlund PM, Bygdeman M, Ekman G. Induction of labor with mifepristone (RU 486). A randomized double‐blind study in post‐term pregnant women with unripe cervices. Acta Obstetricia et Gynecologica Scandinavica Supplement 1994;73(161):Abstract FP50.
-
- Stenlund PM, Ekman G, Aedo AR, Bygdeman M. Induction of labour with mifepristone ‐ a randomized double‐blind study versus placebo. Acta Obstetricia et Gynecologica Scandinavica 1999;78:793‐8. - PubMed
Stitely 2000 {published data only}
-
- Stitely ML, Browning J, Fowler M, Gendron RT, Gherman RB. Outpatient cervical ripening with intravaginal misoprostol. Obstetrics and Gynecology 2000;96(5 Pt 1):684‐8. - PubMed
References to studies excluded from this review
Adewole 1993 {published data only}
-
- Adewole IF, Franklin O, Matiluko AA. Cervical ripening and induction of labour by breast stimulation. African Journal of Medicine and Medical Sciences 1993;22(4):81‐6. - PubMed
Damania 1988 {published data only}
-
- Damania KR, Nanavati MS, Dastur NA, Daftary SN. Breast stimulation for cervical ripening. Journal of Obstetrics and Gynaecology of India 1988;58:663‐5.
Damania 1992 {published data only}
-
- Damania KK, Natu U, Mhatre PN, Mataliya M, Mehta AC, Daftary SN. Evaluation of two methods employed for cervical ripening. Journal of Postgraduate Medicine 1992;38(2):58‐9. - PubMed
Di Lieto 1989 {published data only}
-
- Lieto A, Miranda L, Ardito P, Favale P, Albano G. Changes in the bishop score induced by manual nipple stimulation. A cross‐over randomized study. Clinical and Experimental Obstetrics and Gynecology 1989;16(1):26‐9. - PubMed
Doany 1997 {published data only}
-
- Doany W. Outpatient management of postdate pregnancy with intravaginal prostaglandin E2 and membrane stripping. American Journal of Obstetrics and Gynecology 1996;174(1Pt 2):351.
-
- Doany W, McCarty J. Outpatient management of the uncomplicated postdate pregnancy with intravaginal prostaglandin E2 gel and membrane stripping. Journal of Maternal‐Fetal Medicine 1997;6(2):71‐8. - PubMed
Dorfman 1987 {published data only}
-
- Dorfman P, Lasserre MN, Tetau M. Preparation for childbirth by homeopathy [Preparation a l'accouchement par homeopathie: experimentation en double insu versus placebo]. Cahiers de Biothérapie 1987;94:77‐81.
Elliott 1984 {published data only}
-
- Elliott JP, Flaherty JF. The use of breast stimulation to prevent postdate pregnancy. American Journal of Obstetrics and Gynecology 1984;149(6):628‐32. - PubMed
-
- Elliott JP, Flaherty JF. The use of breast stimulation to ripen the cervix in term pregnancies. American Journal of Obstetrics and Gynecology 1983;145(5):553‐6. - PubMed
Evans 1983 {published data only}
-
- Evans MI, Dougan MB, Moawad AH, Evans WJ, Bryant‐Greenwood GD, Greenwood FC. Ripening of the human cervix with porcine ovarian relaxin. American Journal of Obstetrics and Gynecology 1983;147(4):410‐4. - PubMed
Garry 2000 {published data only}
-
- Garry D, Figueroa R, Guillaume J, Cucco V. Use of castor oil in pregnancies at term. Alternative Therapies in Health and Medicine 2000;6(1):77‐9. - PubMed
Griffin 2003 {published data only}
-
- Griffin C. Outpatient cervical ripening using sequential oestrogen ‐ a randomised controlled pilot study. Australian and New Zealand Journal of Obstetrics and Gynaecology 2003;43:183.
Herabutya 1992 {published data only}
-
- Herabutya Y, Prasertsawat PO, Tongyai T, Isarangura N, Ayudthya N. Prolonged pregnancy: the management dilemma. International Journal of Gynaecology and Obstetrics 1992;37(4):253‐8. - PubMed
Kadar 1990 {published data only}
-
- Kadar N, Tapp A, Wong A. The influence of nipple stimulation at term on the duration of pregnancy. Journal of Perinatology 1990;10(2):164‐6. - PubMed
Kaul 2004 {published data only}
-
- Kaul V, Aggarwal N, Ray P. Membrane stripping versus single dose intracervical prostaglandin gel administration for cervical ripening. International Journal of Gynaecology and Obstetrics 2004;86(3):388‐9. - PubMed
Krammer 1995 {published data only}
-
- Krammer J, O'Brien W, Williams M. Outpatient cervical ripening does not affect gestational age at delivery. American Journal of Obstetrics and Gynecology 1995;172:425.
Magann 1999 {published data only}
-
- Magann EF, Chauhan SP, McNamara MF, Bass JD, Estes CM, Morrison JC. Membrane stripping vs dinoprostone vaginal insert in the management of pregnancies beyond 41 weeks with unfavorable cervix. American Journal of Obstetrics and Gynecology 1998;178(1 Pt 2):S30.
-
- Magann EF, Chauhan SP, McNamara MF, Bass JD, Estes CM, Morrison JC. Membrane sweeping versus dinoprostone vaginal insert in the management of pregnancies beyond 41 weeks with an unfavorable cervix. Journal of Perinatology 1999;19(2):88‐91. - PubMed
Manidakis 1999 {published data only}
-
- Manidakis G, Sifakis S, Orfanoudaki E, Mikelakis G, Prokopakis P, Magou M, et al. Prostaglandin versus stripping of membranes in management of pregnancy beyond 40‐41 weeks [abstract]. European Journal of Obstetrics, Gynecology, and Reproductive Biology 1999;86:S79‐S80.
Moghtadaei 2007 {published data only}
-
- Moghtadaei P. A randomized trial comparing outpatient vaginal isosorbide‐mononitrate versus extra‐amnion saline infusion with concurrent oxytocin for cervical ripening and labor induction in nulliparous women. American Journal of Obstetrics and Gynecology 2007;197(6 Suppl 1):S103.
Ohel 1996 {published data only}
-
- Ohel G, Rahav D, Rothbart H, Ruach M. Randomised trial of outpatient induction of labor with vaginal PGE2 at 40‐41 weeks of gestation versus expectant management. Archives of Gynecology and Obstetrics 1996;258(3):109‐12. - PubMed
Rayburn 1988 {published data only}
-
- Rayburn W, Gosen R, Ramadei C, Woods R, Scott J Jr. Outpatient cervical ripening with prostaglandin E2 gel in uncomplicated postdate pregnancies. American Journal of Obstetrics and Gynecology 1988;158(6 Pt 1):1417‐23. - PubMed
Rezk 2014 {published data only}
-
- Rezk M, Sanad Z, Dawood R, Masood A, Emarh M, Halaby AA. Intracervical foley catheter versus vaginal isosorbid mononitrate for induction of labor in women with previous one cesarean section. Journal of Clinical Gynecology and Obstetrics 2014;3(2):55‐61.
Salamalekis 2000 {published data only}
-
- Salamalekis E, Vitoratos N, Kassanos D, Loghis C, Batalias L, Panayotopoulos N, et al. Sweeping of the membranes versus uterine stimulation by oxytocin in nulliparous women: a randomized controlled trial. Gynecologic and Obstetric Investigation 2000;49(4):240‐3. - PubMed
Salmon 1986 {published data only}
-
- Salmon YM, Kee WH, Tan SL, Jen SW. Cervical ripening by breast stimulation. Obstetrics and Gynecology 1986;67(1):21‐4. - PubMed
Spallicci 2007 {published data only}
-
- Spallicci MD, Chiea MA, Singer JM, Albuquerque PB, Bittar RE, Zugaib M. Use of hyaluronidase for cervical ripening: a randomized trial. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2007;130(1):46‐50. - PubMed
-
- Spallicci MDB, Bittar RE. Randomized double blind study of ripening the cervix with hyaluronidase in term gestations [Estudo clinico aleatorizado com grupo controle e mascaramento duplo da maturacao do colo uterino pela hialuronidase em gestacoes a termo]. Revista Brasileira de Ginecologia e Obstetricia 2003;25(1):67.
Voss 1996 {published data only}
-
- Voss DH, Cumminsky KC, Cook VD, Nethers MS, Spinnato JA, Gall SA. Effect of three concentrations of intracervical prostaglandin E2 gel for cervical ripening. Journal of Maternal‐Fetal Medicine 1996;5(4):186‐93. - PubMed
Ziaei 2003 {published data only}
-
- Ziaei S, Rosebehani N, Kazeminejad A, Zafarghandi S. The effects of intramuscular administration of corticosteroids on the induction of parturition. Journal of Perinatal Medicine 2003;31(2):134‐9. - PubMed
References to studies awaiting assessment
Ascher‐Walsh 2000 {published data only}
-
- Ascher‐Walsh C, Burke B, Baxi L. Outpatient management of prolonged pregnancy with misoprostol (mp): a randomized double‐blind placebo controlled study, prelim data. American Journal of Obstetrics and Gynecology 2000;182(1 Pt 2):S20.
Mostaghel 2009 {published data only}
-
- Mostaghel N, Nakhai F. Outpatient vaginal misoprostol and its effect on post‐term pregnancy. International Journal of Gynaecology and Obstetrics 2009;107(Suppl 2):S586.
Thakur 2005 {published data only}
-
- Thakur V, Dorman E, Sanu L, Harrington K. Mifepristone is an effective ripening agent in postdates primips with cervical length ≥ 2.5cm, but mode of delivery correlates with birthweight: a randomised, placebo controlled double blind study. Ultrasound in Obstetrics & Gynecology 2005;26:452.
Additional references
Alfirevic 2014
Bollapragada 2006b
-
- Bollapragada S, Mackenzie F, Norrie J, Petrou S, Reid M, Greer I, et al. IMOP: randomised placebo controlled trial of outpatient cervical ripening with isosorbide mononitrate (IMN) prior to induction of labour ‐ clinical trial with analyses of efficacy, cost effectiveness and acceptability. BMC Pregnancy and Childbirth 2006;6:25. [DOI: 10.1186/1471-2393-6-25] - DOI - PMC - PubMed
Boulvain 2008
Curtis 1987
-
- Curtis P, Evans S, Resnick J. Uterine hyperstimulation. The need for standard terminology. Journal of Reproductive Medicine 1987;32(2):91‐5. - PubMed
Eddama 2009
-
- Eddama O, Petrou S, Schroeder L, Bollapragada S, Mackenzie F, Norrie J, et al. The cost‐effectiveness of outpatient (at home) cervical ripening with isosorbide mononitrate prior to induction of labour. BJOG: an international journal of obstetrics and gynaecology 2009;116:1196‐1203. - PubMed
Elliott 1992
-
- Elliott JP, Clewell WH, Radin TG. Intracervical prostaglandin E2 gel. Safety for outpatient cervical ripening before induction of labor. Journal of Reproductive Medicine 1992;37(8):713‐6. - PubMed
Ghosh 2016
Glantz 2003
-
- Glantz JC. Labor induction rate variation in upstate New York: what is the difference?. Birth 2003;30(3):168‐74. - PubMed
Guerra 2009
-
- Guerra G, Cecatti J, Souza J, Faundes A, Morais SS, Gulmezoglu AM, et al. Factors and outcomes associated with the induction of labour in Latin America. BJOG: an international journal of obstetrics and gynaecology 2009;116(113):1762‐72. - PubMed
Gülmezoglu 2012
Hapangama 2009
Higgins 2011
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hofmeyr 2009
-
- Hofmeyr GJ, Alfirevic Z, Kelly AJ, Kavanagh J, Thomas J, Neilson JP, et al. Methods for cervical ripening and labour induction in late pregnancy: generic protocol. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD002074] - DOI
Hofmeyr 2010
ISRCTN47736435
-
- ISRCTN47736435. Costs and effects of amniotomy at home for induction of post term pregnancy. isrctn.com/ISRCTN47736435 (first received 9 January 2006).
Kelly 2013
Kirby 2004
-
- Kirby RS. Trends in labor induction in the United States: is it true that what goes up must come down?. Birth 2004;31(2):148‐51. - PubMed
McGill 2007
-
- McGill J, Shetty A. Mifepristone and misoprostol in the induction of labor at term. International Journal of Gynaecology and Obstetrics 2007;96(2):80‐4. - PubMed
Neale 2002
-
- Neale E, Pachulski A, Whiterod S, McGuinness E, Gallagher N, Wallace R. Outpatient cervical ripening prior to induction of labour. Journal of Obstetrics and Gynaecology 2002;22(6):634‐5. - PubMed
NHS 2014‐15
-
- Hospital Episode Statistics Analysis, Health and Social Care Information Centre. Hospital Episode Statistics ‐ NHS Maternity Statistics – England, 2014‐15. http://content.digital.nhs.uk/catalogue/PUB19127/nhs‐mate‐eng‐2014‐15‐su.... London: National Statistics, The Information Centre, 25 November 2015.
Osman 2006
-
- Osman I, MacKenzie F, Norrie J, Murray HM, Greer IA, Norman JE. The "PRIM" study: a randomized comparison of prostaglandin E2 gel with the nitric oxide donor isosorbide mononitrate for cervical ripening before the induction of labor at term. American Journal of Obstetrics and Gynecology 2006;194(4):1012‐21. - PubMed
Ramsey 2005
-
- Ramsey PS, Meyer L, Walkes BA, Harris D, Ogburn PL Jr, Heise RH, et al. Cardiotocographic abnormalities associated with dinoprostone and misoprostol cervical ripening. Obstetrics and Gynecology 2005;105(1):85‐90. - PubMed
Rayburn 2002
-
- Rayburn WF, Zhang J. Rising rates of labor induction: present concerns and future strategies. Obstetrics and Gynecology 2002;100(1):164‐7. - PubMed
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Salvador 2009
-
- Salvador SC, Simpson ML, Cundiff GW. Dinoprostone vaginal insert for labour induction: a comparison of outpatient and inpatient settings. Journal of Obstetrics and Gynaecology Canada 2009;31(11):1028‐34. - PubMed
Sawai 1995
-
- Sawai SK, O'Brien WF. Outpatient cervical ripening. Clinical Obstetrics and Gynecology 1995;38(2):301‐9. - PubMed
Shetty 2005
-
- Shetty A, Burt R, Rice P, Templeton A. Women's perceptions, expectations and satisfaction with induced labour ‐ a questionnaire‐based study. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2005;123(1):56‐61. - PubMed
Smith 2013
Thomas 2001
Thomas 2014
Vogel 2013
WHO 2011
-
- World Health Organization. WHO recommendations for Induction of labour. apps.who.int/iris/bitstream/10665/44531/1/9789241501156_eng.pdf. Geneva: World Health Organization, (accessed prior to 7 August 2017).
References to other published versions of this review
Dowswell 2010
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

