Function-Oriented Studies Targeting Pectenotoxin 2: Synthesis of the GH-Ring System and a Structurally Simplified Macrolactone
- PMID: 28901150
- PMCID: PMC5633828
- DOI: 10.1021/acs.orglett.7b02435
Function-Oriented Studies Targeting Pectenotoxin 2: Synthesis of the GH-Ring System and a Structurally Simplified Macrolactone
Abstract
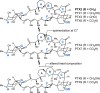
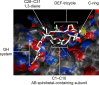
A chemical foundation for function-oriented studies of pectenotoxin 2 (PTX2) is described. A synthesis of the bicyclic GH-system, and the design and synthesis of a PTX2-analogue, is presented. While maintaining critical features for actin binding, and lacking the Achilles' heel for the natural product's anticancer activity (the AB-spiroketal), this first-generation analogue did not possess the anticancer properties of PTX2, an observation that indicates the molecular significance of features present in the natural product's CDEF-tetracycle.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
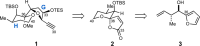



References
-
- Yasumoto T.; Murata M.; Oshima Y.; Sano M.; Matsumoto G. K.; Clardy J. Tetrahedron 1985, 41, 1019.10.1016/S0040-4020(01)96469-5. - DOI
-
- Chae H.-D.; Choi T.-S.; Kim B.-M.; Jung J. H.; Bang Y.-J.; Shin D. Y. Oncogene 2005, 24, 4813.10.1038/sj.onc.1208640. - DOI - PubMed
- Jung J. H.; Sim C. J.; Lee C.-O. J. Nat. Prod. 1995, 58, 1722.10.1021/np50125a012. - DOI - PubMed
- Shin D. Y.; Kim G. Y.; Kim N. D.; Jung J. H.; Kim S.-K.; Hang H. S.; Choi Y. H. Oncol. Rep. 2008, 19, 517.10.3892/or.19.2.517. - DOI - PubMed
- Chae H. D.; Kim B. M.; Yun U. J.; Shin D. Y. Oncogene 2008, 27, 4115.10.1038/onc.2008.46. - DOI - PubMed
-
For action of other actin-targeting cytotoxic agents, see:
- Lee Y.-J.; Tsai C.-H.; Hwang J.-J.; Chiu S.-J.; Sheu T.-J.; Keng P. C. Int. J. Oncol. 2009, 34, 581. - PubMed
-
-
For the total synthesis of PTX2, see:
- Fujiwara K.; Suzuki Y.; Koseki N.; Aki Y.; Kikuchi Y.; Murata S.; Yamamoto F.; Kawamura M.; Norikura R.; Matsue H.; Murai A.; Katoono R.; Kawai H.; Suzuki T. Angew. Chem., Int. Ed. 2014, 53, 780.10.1002/anie.201308502. - DOI - PubMed
-
For total syntheses of PTX4 and PTX8, see:
- Evans D. A.; Rajapakse H. A.; Stenkamp D. Angew. Chem., Int. Ed. 2002, 41, 4569.10.1002/1521-3773(20021202)41:23<4569::AID-ANIE4569>3.0.CO;2-V. - DOI - PubMed
- Evans D. A.; Rajapakse H. A.; Chiu A.; Stenkamp D. Angew. Chem., Int. Ed. 2002, 41, 4573.10.1002/1521-3773(20021202)41:23<4573::AID-ANIE4573>3.0.CO;2-S. - DOI - PubMed
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

