PPAR-δ Agonist With Mesenchymal Stem Cells Induces Type II Collagen-Producing Chondrocytes in Human Arthritic Synovial Fluid
- PMID: 28901183
- PMCID: PMC5680970
- DOI: 10.1177/0963689717720278
PPAR-δ Agonist With Mesenchymal Stem Cells Induces Type II Collagen-Producing Chondrocytes in Human Arthritic Synovial Fluid
Abstract
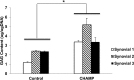
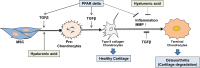
Osteoarthritis (OA) is an inflammatory joint disease characterized by degeneration of articular cartilage within synovial joints. An estimated 27 million Americans suffer from OA, and the population is expected to reach 67 million in the United States by 2030. Thus, it is urgent to find an effective treatment for OA. Traditional OA treatments have no disease-modifying effect, while regenerative OA therapies such as autologous chondrocyte implantation show some promise. Nonetheless, current regenerative therapies do not overcome synovial inflammation that suppresses the differentiation of mesenchymal stem cells (MSCs) to chondrocytes and the expression of type II collagen, the major constituent of functional cartilage. We discovered a synergistic combination that overcame synovial inflammation to form type II collagen-producing chondrocytes. The combination consists of peroxisome proliferator-activated receptor (PPAR) δ agonist, human bone marrow (hBM)-derived MSCs, and hyaluronic acid (HA) gel. Interestingly, those individual components showed their own strong enhancing effects on chondrogenesis. GW0742, a PPAR-δ agonist, greatly enhanced MSC chondrogenesis and the expression of type II collagen and glycosaminoglycan (GAG) in hBM-MSC-derived chondrocytes. GW0742 also increased the expression of transforming growth factor β that enhances chondrogenesis and suppresses cartilage fibrillation, ossification, and inflammation. HA gel also increased MSC chondrogenesis and GAG production. However, neither GW0742 nor HA gel could enhance the formation of type II collagen-producing chondrocytes from hBM-MSCs within human OA synovial fluid. Our data demonstrated that the combination of hBM-MSCs, PPAR-δ agonist, and HA gel significantly enhanced the formation of type II collagen-producing chondrocytes within OA synovial fluid from 3 different donors. In other words, the novel combination of PPAR-δ agonist, hBM-MSCs, and HA gel can overcome synovial inflammation to form type II collagen cartilage within human OA synovial fluid. This novel articularly injectable formula could improve OA treatment in the future clinical application.
Keywords: PPAR-δ agonist; hyaluronic acid; mesenchymal stem cells; osteoarthritis; synovial inflammation; type II collagen.
Conflict of interest statement
Figures





References
-
- Knutsen G, Drogset JO, Engebretsen L, Grontvedt T, Isaksen V, Ludvigsen TC, Roberts S, Solheim E, Strand T, Johansen O. A randomized trial comparing autologous chondrocyte implantation with microfracture. Findings at five years. J Bone Joint Surg Am. 2007;89(10):2105–2112. - PubMed
-
- McNickle AG, L’Heureux DR, Yanke AB, Cole BJ. Outcomes of autologous chondrocyte implantation in a diverse patient population. Am J Sports Med. 2009;37(7):1344–1350. - PubMed
-
- Pelttari K, Steck E, Richter W. The use of mesenchymal stem cells for chondrogenesis. Injury. 2008;39(suppl 1):S58–S65. - PubMed
-
- Farkouh ME, Kirshner H, Harrington RA, Ruland S, Verheugt FW, Schnitzer TJ, Burmester GR, Mysler E, Hochberg MC, Doherty M, et al. ; TARGET Study Group. Comparison of lumiracoxib with naproxen and ibuprofen in the Therapeutic Arthritis Research and Gastrointestinal Event Trial (TARGET), cardiovascular outcomes: randomised controlled trial. Lancet. 2004;364(9435):675–684. - PubMed
-
- Lazzaroni M, Bianchi Porro G. Gastrointestinal side-effects of traditional non-steroidal anti-inflammatory drugs and new formulations. Aliment Pharmacol Ther. 2004;20(Suppl 2):48–58. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

