Recipient Adipose-Derived Stem Cells Enhance Recipient Cell Engraftment and Prolong Allotransplant Survival in a Miniature Swine Hind-Limb Model
- PMID: 28901186
- PMCID: PMC5680982
- DOI: 10.1177/0963689717724534
Recipient Adipose-Derived Stem Cells Enhance Recipient Cell Engraftment and Prolong Allotransplant Survival in a Miniature Swine Hind-Limb Model
Abstract
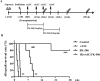
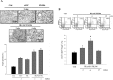
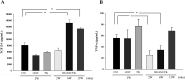
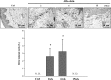
Donor mesenchymal stem cells (MSCs) could prolong vascularized composite allotransplantation (VCA) survival in our previous studies. However, recipient adipose tissue is easier to harvest than donor tissue for preconditioning modulation. Hence, this study investigated the efficacy of recipient autologous adipose-derived stem cells (rADSCs) for VCA survival. The heterotopic hind-limb transplantation from female donor to male recipient was performed in outbred miniature swine. Group I ( n = 6) was untreated controls. Group II ( n = 4) obtained rADSCs infusions (given on weeks 0, +1, +2, and +3). Group III ( n = 4) obtained tacrolimus (FK506, weeks 0 to +4). Group IV ( n = 8) received irradiation (IR; day -1), FK506 (weeks 0 to +4), and rADSC infusions (weeks 0, +1, +2, and +3). The results revealed treatment with multiple injections of rADSCs along with IR and FK506 resulted in a statistically significant increase in allograft survival. The percentage of CD4+/CD25+/Foxp3+ regulatory T cells were significantly increased in the rADSC-IR-FK506 group as compared to controls. Analysis of recipient peripheral blood revealed that transforming growth factor β1 (TGFβ1) was significantly increased in the rADSC-IR-FK506 group. The polymerase chain reaction (PCR) analysis and immunohistochemical staining showed recipient sex-determining region of Y (SRY) chromosome gene expression existed in donor allotissues in the rADSC-IR-FK506 group. These results indicate that rADSCs in addition to IR and transient immunosuppressant could prolong allotransplant survival, modulate T-cell regulation, and enhance recipient cell engraftment into the allotransplant tissues.
Keywords: adipose-derived stem cells; graft rejection; regulatory T cell; vascularized composite allotransplantation.
Conflict of interest statement
Figures

Similar articles
-
Mesenchymal stem cells prolong composite tissue allotransplant survival in a swine model.Transplantation. 2009 Jun 27;87(12):1769-77. doi: 10.1097/TP.0b013e3181a664f1. Transplantation. 2009. PMID: 19543052
-
Prolongation of composite tissue allotransplant survival by treatment with bone marrow mesenchymal stem cells is correlated with T-cell regulation in a swine hind-limb model.Plast Reconstr Surg. 2011 Feb;127(2):569-579. doi: 10.1097/PRS.0b013e318200a92c. Plast Reconstr Surg. 2011. PMID: 21285761
-
Modulation of immune response and T-cell regulation by donor adipose-derived stem cells in a rodent hind-limb allotransplant model.Plast Reconstr Surg. 2011 Dec;128(6):661e-672e. doi: 10.1097/PRS.0b013e318230c60b. Plast Reconstr Surg. 2011. PMID: 22094768
-
Adipose-derived cellular therapies in solid organ and vascularized-composite allotransplantation.Curr Opin Organ Transplant. 2017 Oct;22(5):490-498. doi: 10.1097/MOT.0000000000000452. Curr Opin Organ Transplant. 2017. PMID: 28873074 Review.
-
Modern trends in lipomodeling.GMS Interdiscip Plast Reconstr Surg DGPW. 2017 Apr 3;6:Doc06. doi: 10.3205/iprs000108. eCollection 2017. GMS Interdiscip Plast Reconstr Surg DGPW. 2017. PMID: 28401032 Free PMC article. Review.
Cited by
-
Clinical and preclinical tolerance protocols for vascularized composite allograft transplantation.Arch Plast Surg. 2021 Nov;48(6):703-713. doi: 10.5999/aps.2021.00927. Epub 2021 Nov 15. Arch Plast Surg. 2021. PMID: 34818720 Free PMC article.
-
Toward transplantation tolerance with adipose tissue-derived therapeutics.Front Immunol. 2023 Apr 28;14:1111813. doi: 10.3389/fimmu.2023.1111813. eCollection 2023. Front Immunol. 2023. PMID: 37187733 Free PMC article. Review.
-
The transplantation of particulated juvenile allograft cartilage and synovium for the repair of meniscal defect in a lapine model.J Orthop Translat. 2022 Mar 4;33:72-89. doi: 10.1016/j.jot.2022.02.004. eCollection 2022 Mar. J Orthop Translat. 2022. PMID: 35281522 Free PMC article.
-
[Research progress of adipose-derived stem cells in promoting the repair of peripheral nerve injury].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2020 Aug 15;34(8):1059-1064. doi: 10.7507/1002-1892.201910009. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2020. PMID: 32794679 Free PMC article. Review. Chinese.
-
Adipose-derived cellular therapies prolong graft survival in an allogenic hind limb transplantation model.Stem Cell Res Ther. 2021 Jan 29;12(1):94. doi: 10.1186/s13287-021-02162-7. Stem Cell Res Ther. 2021. PMID: 33514430 Free PMC article.
References
-
- Dubernard JM, Devauchelle B. Face transplantation. Lancet. 2008;372(9639):603–604. - PubMed
-
- Jones JW, Gruber SA, Barker JH, Breidenbach WC. Successful hand transplantation. One-year follow-up. Louisville hand transplant team. N Engl J Med. 2000;343(7):468–473. - PubMed
-
- Lee WP. Composite tissue transplantation: more science and patience needed. Plast Reconstr Surg. 2001;107(4):1066–1070. - PubMed
-
- Madani H, Hettiaratchy S, Clarke A, Butler PE. Immunosuppression in an emerging field of plastic reconstructive surgery: composite tissue allotransplantation. J Plast Reconstr Aesthet Surg. 2008;61(3):245–249. - PubMed
-
- Siemionow MZ, Kulahci Y, Bozkurt M. Composite tissue allotransplantation. Plast Reconstr Surg. 2009;124(Suppl 6):e327–e339. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

