Cryopreservation of Hepatocyte Microbeads for Clinical Transplantation
- PMID: 28901189
- PMCID: PMC5680969
- DOI: 10.1177/0963689717720050
Cryopreservation of Hepatocyte Microbeads for Clinical Transplantation
Abstract
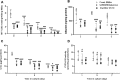
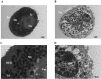
Intraperitoneal transplantation of hepatocyte microbeads is an attractive option for the management of acute liver failure. Encapsulation of hepatocytes in alginate microbeads supports their function and prevents immune attack of the cells. Establishment of banked cryopreserved hepatocyte microbeads is important for emergency use. The aim of this study was to develop an optimized protocol for cryopreservation of hepatocyte microbeads for clinical transplantation using modified freezing solutions. Four freezing solutions with potential for clinical application were investigated. Human and rat hepatocytes cryopreserved with University of Wisconsin (UW)/10% dimethyl sulfoxide (DMSO)/5% (300 mM) glucose and CryoStor CS10 showed better postthawing cell viability, attachment, and hepatocyte functions than with histidine-tryptophan-ketoglutarate/10% DMSO/5% glucose and Bambanker. The 2 freezing solutions that gave better results were studied with human and rat hepatocytes microbeads. Similar effects on cryopreserved microbead morphology (external and ultrastructural), viability, and hepatocyte-functions post thawing were observed over 7 d in culture. UW/DMSO/glucose, as a basal freezing medium, was used to investigate the additional effects of cytoprotectants: a pan-caspase inhibitor (benzyloxycarbonyl-Val-Ala-dl-Asp-fluoromethylketone [ZVAD]), an antioxidant (desferoxamine [DFO]), and a buffering and mechanical protectant (human serum albumin [HSA]) on RMBs. ZVAD (60 µM) had a beneficial effect on cell viability that was greater than with DFO (1 mM), HSA (2%), and basal freezing medium alone. Improvements in the ultrastructure of encapsulated hepatocytes and a lower degree of cell apoptosis were observed with all 3 cytoprotectants, with ZVAD tending to provide the greatest effect. Cytochrome P450 activity was significantly higher in the 3 cytoprotectant groups than with fresh microbeads. In conclusion, developing an optimized cryopreservation protocol by adding cytoprotectants such as ZVAD could improve the outcome of cryopreserved hepatocyte microbeads for future clinical use.
Keywords: acute liver failure; alginate encapsulation; apoptosis; clinical grade; cryopreservation; cytoprotectants; hepatocyte microbeads.
Conflict of interest statement
Figures





Similar articles
-
Alginate microencapsulated hepatocytes optimised for transplantation in acute liver failure.PLoS One. 2014 Dec 1;9(12):e113609. doi: 10.1371/journal.pone.0113609. eCollection 2014. PLoS One. 2014. PMID: 25438038 Free PMC article.
-
A novel method of cryopreservation of rat and human hepatocytes by using encapsulation technique and possible use for cell transplantation.Cell Transplant. 2005;14(9):609-20. doi: 10.3727/000000005783982710. Cell Transplant. 2005. PMID: 16405071
-
Alginate microencapsulated human hepatocytes for the treatment of acute liver failure in children.J Hepatol. 2020 May;72(5):877-884. doi: 10.1016/j.jhep.2019.12.002. Epub 2019 Dec 13. J Hepatol. 2020. PMID: 31843649
-
Cryopreservation of isolated human hepatocytes for transplantation: State of the art.Cryobiology. 2006 Oct;53(2):149-59. doi: 10.1016/j.cryobiol.2006.05.004. Epub 2006 Jun 21. Cryobiology. 2006. PMID: 16793034 Review.
-
An optimised method for cryopreservation of human hepatocytes.Methods Mol Biol. 2009;481:25-34. doi: 10.1007/978-1-59745-201-4_3. Methods Mol Biol. 2009. PMID: 19096796 Review.
Cited by
-
Winter is coming: the future of cryopreservation.BMC Biol. 2021 Mar 24;19(1):56. doi: 10.1186/s12915-021-00976-8. BMC Biol. 2021. PMID: 33761937 Free PMC article. Review.
-
Clinical Hepatocyte Transplantation: What Is Next?Curr Transplant Rep. 2017 Dec;4(4):280-289. doi: 10.1007/s40472-017-0165-6. Epub 2017 Oct 14. Curr Transplant Rep. 2017. PMID: 29732274 Free PMC article.
-
Cryopreserved cGMP-compliant human pluripotent stem cell-derived hepatic progenitors rescue mice from acute liver failure through rapid paracrine effects on liver cells.Stem Cell Res Ther. 2024 Mar 12;15(1):71. doi: 10.1186/s13287-024-03673-9. Stem Cell Res Ther. 2024. PMID: 38475825 Free PMC article.
-
Freezing biological organisms for biomedical applications.Smart Med. 2022 Dec 27;1(1):e20220034. doi: 10.1002/SMMD.20220034. eCollection 2022 Dec. Smart Med. 2022. PMID: 39188743 Free PMC article. Review.
-
A New High Throughput Screening Platform for Cell Encapsulation in Alginate Hydrogel Shows Improved Hepatocyte Functions by Mesenchymal Stromal Cells Co-encapsulation.Front Med (Lausanne). 2018 Aug 9;5:216. doi: 10.3389/fmed.2018.00216. eCollection 2018. Front Med (Lausanne). 2018. PMID: 30140676 Free PMC article.
References
-
- Khuroo M, Khuroo M, Farahat K. Molecular adsorbent recirculating system for acute and acute-on-chronic liver failure: a meta-analysis. Liver Transpl. 2004;10(9):1099–1106. - PubMed
-
- Oppert M, Rademacher S, Petrasch K, Jörres A. Extracorporeal liver support therapy with Prometheus in patients with liver failure in the intensive care unit. Ther Apher Dial. 2009;13(5):426–430. - PubMed
-
- Struecker B, Raschzok N, Sauer I. Liver support strategies: cutting-edge technologies. Nat Rev Gastroenterol Hepatol. 2014;11(3):166–176. - PubMed
-
- Mai G, Nguyen T, Morel P, Mei J, Andres A, Bosco D, Baertschiger R, Toso C, Berney T, Majno P, et al. Treatment of fulminant liver failure by transplantation of microencapsulated primary or immortalized xenogeneic hepatocytes. Xenotransplantation. 2005;12(6):457–464. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

